This page is part of the Pharmaceutical Quality (Industry) (v1.0.0-ballot: STU1 Ballot 1) based on FHIR (HL7® FHIR® Standard) v5.0.0. The current version which supersedes this version is 1.0.0. For a full list of available versions, see the Directory of published versions
Details about test methods used to analyze substances and products, including compendial and non-compendial testing.
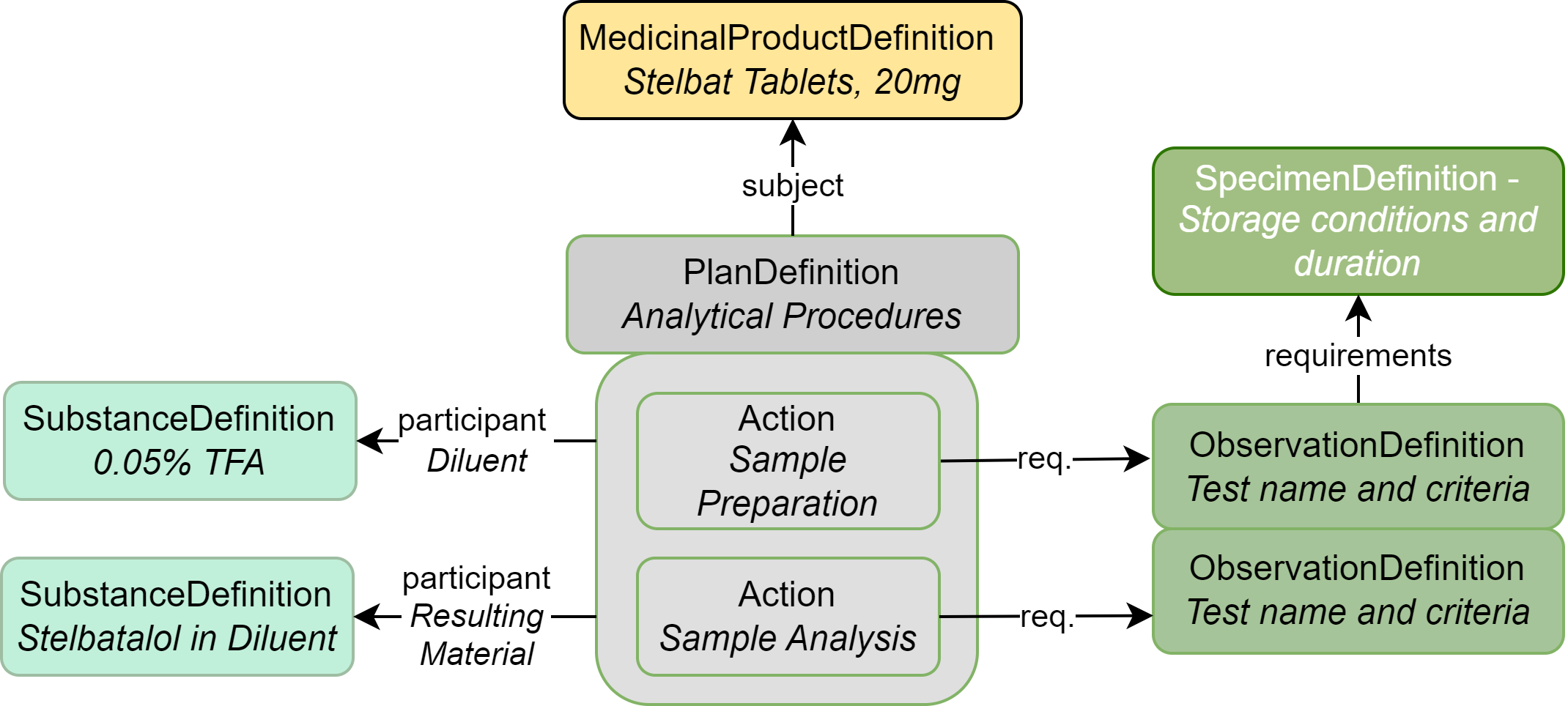 |
| MedicinalProductDefinition | The drug product (Stelbat tablets, 20mg) |
| SubstanceDefinition | Substances used during the analysis process |
| PlanDefinition | Describes the analysis process |
| SpecimenDefinition | Describes the storage conditions and duration for each test |
| Observation Definition | Each individual analysis test and acceptance criteria; also used to group tests |
eCTD section synthetic source data samples (PDF):
XML and JSON examples of synthetic CTD data:
HTML presentation examples of synthetic CTD data:
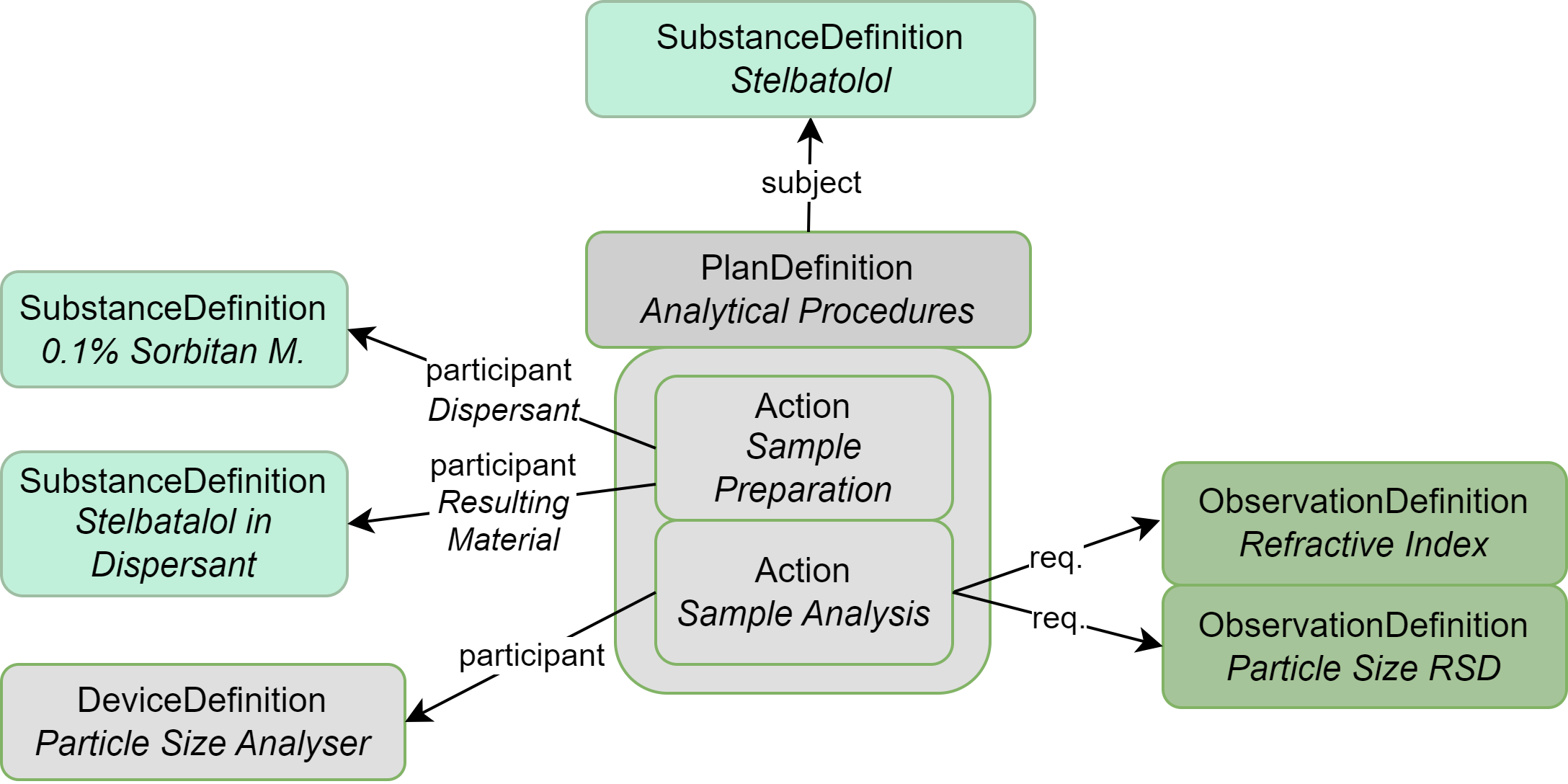 |
| SubstanceDefinition | Substances to be analyzed and also ones used during the analysis process |
| PlanDefinition | Describes the analysis process |
| DeviceDefinition | Devices/equipment used during testing |
| SpecimenDefinition | Describes the storage conditions and duration for each test |
| Observation Definition | Each individual analysis test and acceptance criteria; also used to group tests |
CTD section samples (PDF):
XML and JSON examples of synthetic CTD data:
HTML rendering of synthetic CTD data: