This page is part of the Pharmaceutical Quality (Industry) (v1.0.0-ballot: STU1 Ballot 1) based on FHIR (HL7® FHIR® Standard) v5.0.0. The current version which supersedes this version is 1.0.0. For a full list of available versions, see the Directory of published versions
Details about compatibility studies and results.
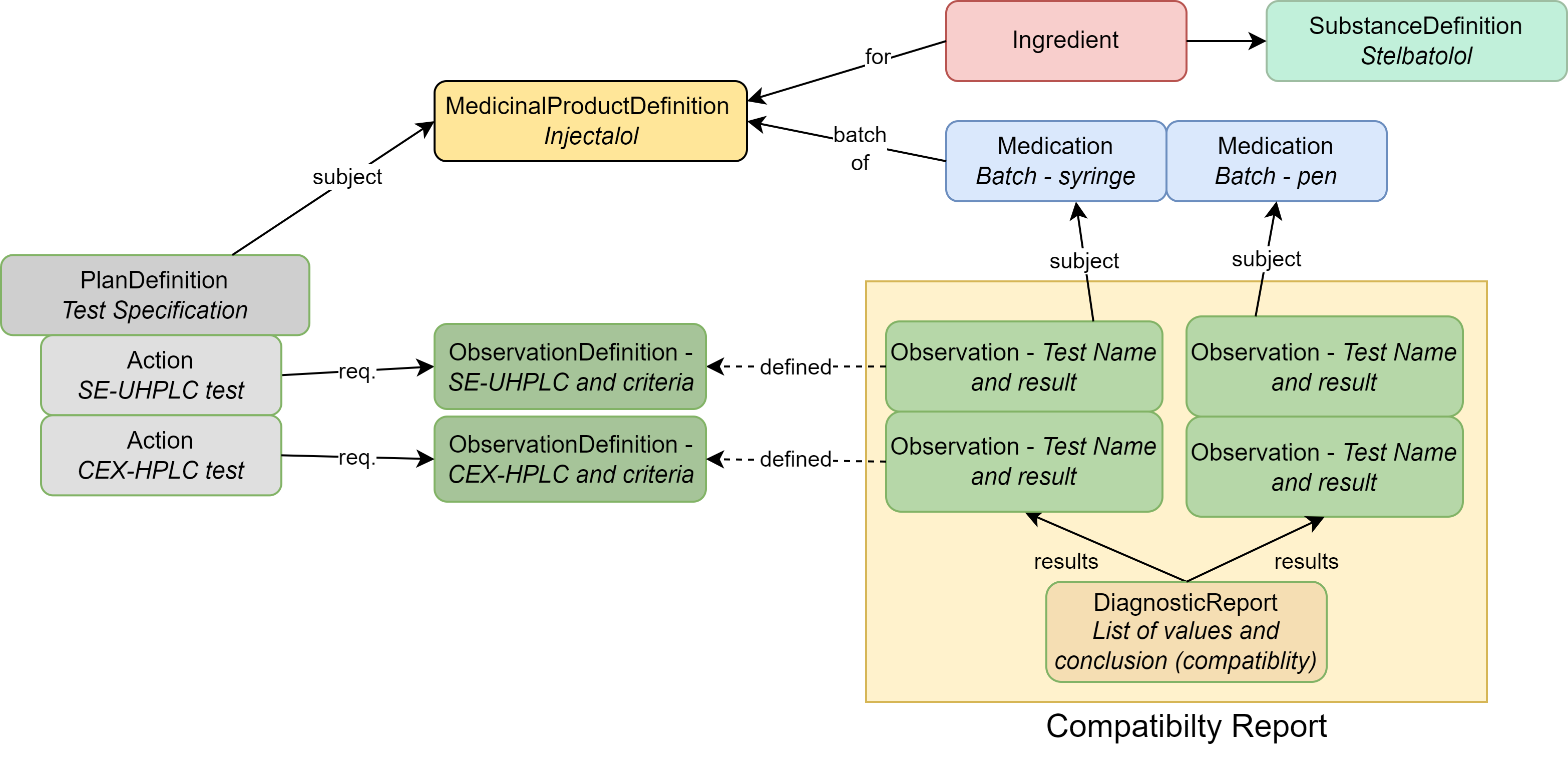 |
| MedicinalProductDefinition | The drug product (Stelbat tablets, 20mg) |
| Medication | Describes the batches that underwent testing |
| Ingredient | The active ingredient (stelbatalol) or the ingredients that make up the drug product |
| SubstanceDefinition | Chemical or biological details about substance(s) associated with the ingredient |
| PlanDefinition | Describes the compatibility analysis protocol |
| ObservationDefinition | Each individual test and acceptance criteria; also used to group tests |
| Observation | The results of a specific test mentioned in the ObservationDefinition |
| DiagnosticReport | Contains all results as a group and captures conclusions |
CTD section samples (PDF):
XML and JSON examples of synthetic CTD data:
HTML rendering of synthetic CTD data: