This section defines the "core" profiles and concepts that would be expected to be present in most genetic reports, regardless of type and how those profiles relate to each other. Concepts covered include the genomics report itself and the high-level categories of observations and other elements that make up the report, such as patient, specimen, variants, haplotypes, genotypes, etc.
1.2.1 Diagnostic Report

The diagnostic report is the focus of all genetic reporting. It conveys metadata about the overall report (what kind of report it was, when it was written, who wrote it, final vs. draft, etc.). It also typically includes a rendered version for review by a clinician. It also groups together all "relevant" information found as part of the genetic analysis. (Rules for relevancy will be dependent the type of testing ordered, the indicated reason for testing and the policies of the lab.) The information found is expressed as FHIR Observations. These observations fall into one of four categories:
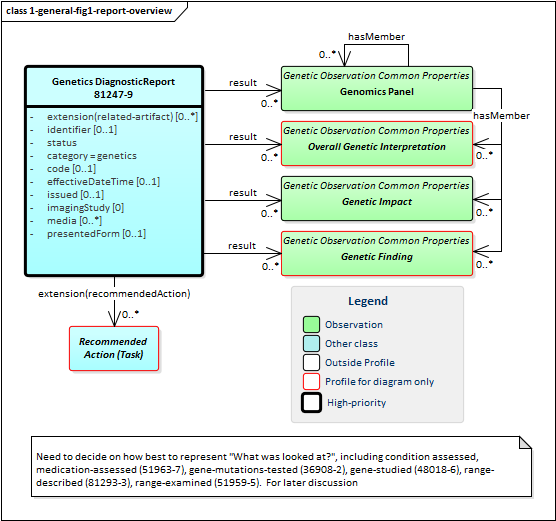
Figure 1: Genetic Report Overview
(Profile links:
Genetics DiagnosticReport,
Genetics Panel,
Overall Genetic Interpretation (see Figure 6),
Genetic Impact,
Genetic Finding (see Figure 5)
)
| Genetic Interpretations |
These are high-level assessments of the result of the genetic testing, generally expressed based on the question asked in the initiating diagnostic service request. For example, "Were any deletions or duplications found?" |
| Genetic Impacts |
These represent [[phenotypic assertions]] about the patient based on the genetic test results. For example, "Patient may have increased susceptibility to heart attacks" |
| Genetic Assertions |
These are observations about the specimen's genetic characteristics. For example, a chromosomal abnormality, genotype, haplotype or variant that was detected. |
One area yet to be resolved is how to best capture "what was looked for" - i.e. the genes/diseases/medications/etc. that were evaluated as part of testing. Because FHIR resources are intended to be stand-alone, any of this information that is relevant to a particular Observation needs to be conveyed as part of that Observation. The work group is still considering how best to reflect this information in the model.
In addition to the observations included in the report, some reports might also recommend specific actions be taken, such as genetic counseling, re-testing, adjusting drug dosages, etc. - driven by the results found. These are covered by the Recommended Action category and are expressed using FHIR's Task resource.
Various Observations including vital signs, lab information, assessments, genetic information, etc. result in different risk assessment. This resource captures predicted outcomes for a patient or population on the basis of source information. These are covered by DiagnosticReport risk which provides a link to an assessment of prognosis or risk as informed by the diagnostic results (For example, genetic results and possibly by patient genetic family history information). This extension is used to when need RiskAssessment as an alternate choice for Observation.hasMember or DiagnosticReport.result.
As shown in the diagram above, all of the observations may hang directly off of the diagnostic report However, they can also be part of a panel. In this version of the specification, no guidance is provided on when or if panels should be used. This is left up to the discretion of the reporting lab. Observations might be organized on the basis of subject, specimen, chromosome, gene, condition/disease, medication or other appropriate measure. The recursive "hasMember" relationship on panel supports a nested tree-structure of panels if appropriate, though more than two levels of panels is likely excessive.
Any organization of observations into panels or sub-panels is purely for navigation and presentation purposes. It carries no additional "meaning". Each observation can be interpreted on its own without knowing the associated panel or sub-panel. The organization of observations in panels does not assert any relationship between observations.
However, it is possible for relationships to exist between the different observation components of a genetic report. Such relationships are asserted directly on one of the affected observations. Some of these relationship types are defined on the basis of the high-level observation category the observation belongs to. Others will be defined for narrower categories or explicit observation types. The high-level category relationships are shown in the following diagram:
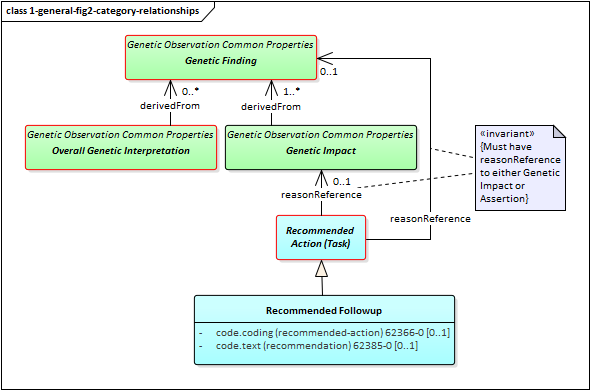
Figure 2: Genetic Report Category Relationships
(Profile links:
Genetics Panel,
Genetic Finding (see Figure 5),
Genetic Impact
)
The relationships between categories are as follows:
-
Genetic Interpretations can be "derived from" Genetic Assertions. For example, an interpretation asserting that "deletions or duplications were found" might be supported by observations of variants that contain deletions and/or duplications.
-
Genetic Impacts MUST be "derived from" Genetic Assertions. For example, in a genetic report, it's not acceptable to assert "patient is an increased metabolizer of drug X" without also indicating the variant, haplotype or genotype found that supports that assertion.
- Every Recommended Action will have a reason relationship to either a Genetic Interpretation or Genetic Assertion. For example: a recommendation to increase the dosage of a medication might be tied to a genetic interpretation indicating that the patient is an increased metabolizer of that medication; an assertion that re-testing should be performed on a variant that was detected but had low quality metrics for certainty.
This diagram also shows a specific example of a Recommended Action - the Recommended Followup which includes suggestions for confirmatory testing, additional testing and/or genetic counselling.
1.2.2 General Relationships

To allow searching and appropriate navigation, the diagnostic report, observations and tasks cannot stand on their own. They need to be related to the associated patient and/or specimen, the order that initiated the testing, the lab that performed the testing, etc. FHIR design principles dictate that these associations be present on every resource instance. That's because each resource could be accessed on its own as part of a query response, embedded in a document or message, passed to a decision support engine, etc. However, this is still relatively lightweight because the information is included by reference only.
The following diagram shows the relationships between the diagnostic report, observations and other elements used in the profile. Note that there is no expectation that all relationships will point to the same instances. In some cases, a genetic report may involve multiple patients or multiple specimens.
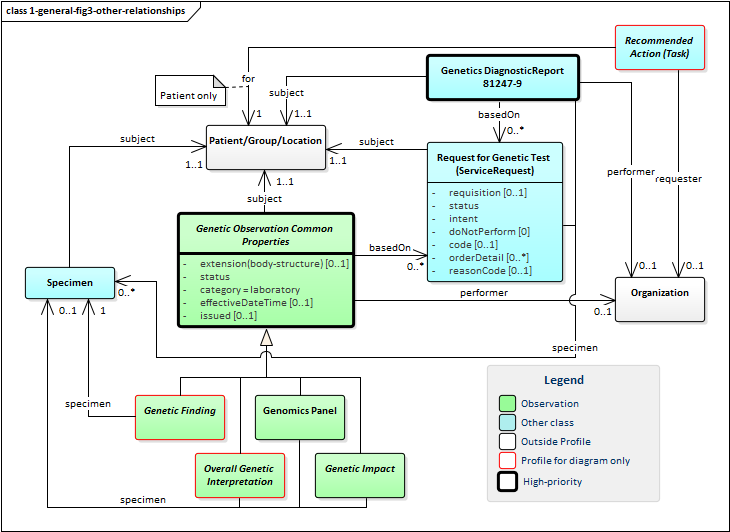
Figure 3: Genetic Report Other Relationships
(Profile links:
Recommended Action (see Figure 2),
Genetics DiagnosticReport,
Request for Genetic Test,
Genetic Observation Common Properties,
Specimen,
Genetic Finding (see Figure 5),
Overall Genetic Interpretation (see Figure 6),
Genomics Panel,
Genetic Impact
)
A few key points to take from this diagram:
-
Orders for genetic testing and the reports resulting from them can be associated to patients, to specimens or both.
- Specimens may be linked to a specific subject, but they can also be stand-alone. For example, genetic testing of a sample swabbed from a counter-top.
- Family member history and tasks are always associated with a patient, not a specimen.
- All genetic observations are derived from a common abstract profile that asserts they should have a category, effective date, issued date and status.
- The effective date is the date the genetic specimen was collected and the issued date is when the observation was performed.
- Of the different types of observations, Genetic Findings all have exactly one specimen. The remainder might be associated with a specimen, but might not. Observations may also be associated with a particular BodyStructure, such as a fetus, tumor or lesion.
- Genetic reports and observations can be tied to multiple "orders" - this is because each test requested is handled as a separate order. All tests ordered as part of a single requisition are linked by the
requisition identifier.
- The Request for Genetic Test typically represents a clinician order. However, it can also represent a lab-side filler order, a reflex order or even a plan or recommendation. These uses are distinguished via the
intent element.
- The primary test to perform is captured in ServiceRequest.code. However, Qualifications on what variants, medications, diseases and other aspects to search on can be conveyed using the
orderDetail element
Orders for genetic tests can point to other sources of information used to support the analysis performed as part of genetic testing. Genetic reports can refer to this the information that was considered as part of the report - whether provided as part of the order or made available subsequently by the patient or clinicians or otherwise retrieved. Figure 4 (below) shows these relationships, which can be to various Observations, FamilyMemberHistory records (including records that comply with Family member history for genetics analysis and RiskAssessments. In some cases, the lab or other reporting organization may generate risk assessments as part of their reports.
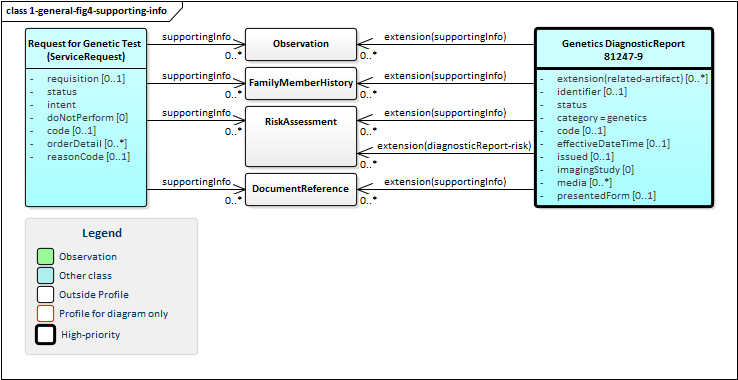
Figure 4: Genetic Report Supporting Information
(Profile links:
Genetics DiagnosticReport,
Request for Genetic Test
)
(Profile links:
Recommended Action (see Figure 2),
Genetics DiagnosticReport,
Request for Genetic Test,
Genetic Observation Common Properties,
Specimen,
Genetic Finding (see Figure 5),
Overall Genetic Interpretation (see Figure 6),
Genomics Panel,
Genetic Impact,
)
1.2.3 Genetic Findings

The primary focus of genetic testing is making Genetic Findings. These are the fine and coarse-grained descriptions of a specimen's genetic characteristics. It is this information that leads to the actionable Genetic Impacts and the Overall Interpretations for the report.
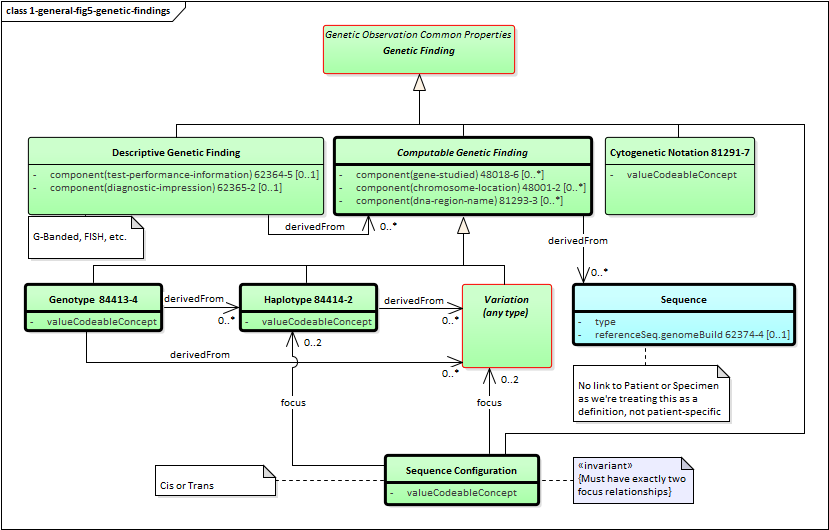
Figure 5: Genetic Findings
(Profile links:
Recommended Action (see Figure 2),
Descriptive Genetic Finding,
Computable Genetic Finding,
Cytogenic Notation,
Genotype,
Haplotype,
Variation (see Sequenced Variants Figure 1),
Sequence,
Sequence Configuration
)
Genetic findings can be broken into two categories: those where the results are expressed as discrete coded data elements (computable findings) and those where the results are primarily expressed as human narrative (non-computable findings). Both types of findings can support the determination of genetic impacts, but only computable findings can be used with automated decision support.
Computable findings can be subdivided into three types of observations:
-
Genotypes describe combinations of genetic variations that together are associated with a particular phenotype - i.e. a specific physical, behavioral or risk-associated difference associated with the organism whose specimen was tested.
-
Haplotypes describe a set of genetic variations that appear on a single strand of DNA - and which are therefore typically inherited together
-
Variants are specific differences or combinations of differences between parts of one or more specimen sequences and the equivalent portions of the reference sequence(s) for that organism.
These categories of observations have relationships. Haplotypes can be identified based on the presence of variants. Genotypes can be identified based on the presence of haplotypes and/or variants. All three can be expressed as a combination of one or more sequences.
1.2.4 Genetic Interpretations

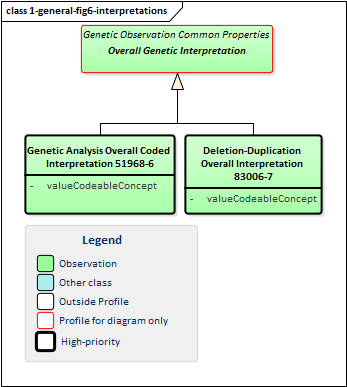
Figure 6: Genetic Report Interpretations
(Profile links:
Genetic Analysis Overall Coded Interpretation,
Deletion-Duplication Overall Interpretation
)
Overall interpretations are high-level summary observations that apply to the whole report. Typically there will only be one of each type - or at least one of each type for a given service request. Their purpose is to answer the question "Did you find anything when you did the test I asked you to do?". There are two high-level intepretations that apply to most genetic reports:
-
Genetic Analysis Overall Coded Interpretation is typically used when the genetic test was looking for a particular genetically-based disease. It allows indication of whether the disease was found or not
-
Deletion Duplication Overall Interpretation merely allows indication of whether deletions or duplications were identified in the region(s) where analysis was requested. (This isn't useful unless testing is constrained to specific regions as almost everyone has deletions and duplications somewhere.)
1.2.5 Genetic Impacts

At present, impacts are noted as explicit observations about the patient/subject. However, it's not clear this is the correct approach. The work group is evaluating introducing a new resource that allows conveying "knowledge" about a variant in a patient-independent way. This would allow saying "this variant is associated with an increase risk of cardiovascular disease" rather than "based on this variant, the patient is at an increased risk of cardiovascular disease", which isn't necessarily a determination the reporting organization may wish to assert. Feedback is welcome.
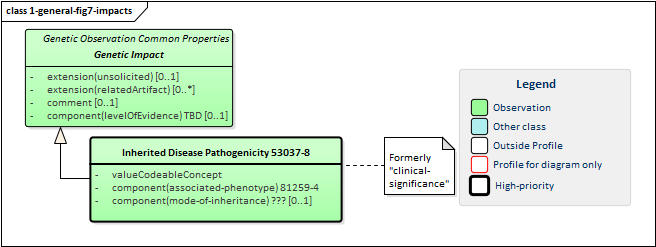
Figure 7: Genetic Report Impacts
(Profile links:
Genetic Impact,
Inherited Disease Pathogenicity
)
Genetic impacts are assertions of likely effects genetic results on the patient, tumor or other subject. All impacts inherit a common set of elements:
-
unsolicited allows distinction of impacts where assessment was specifically asked for as part of the original order and those provided as "additional information" by the reporting organization
-
relatedArtifact supports conveying references to citations, supporting documentation and other information relevant to the assertion of the impact
-
comment contains additional detail and possibly qualification of the asserted impact
-
levelOfEvidence indicates the strength of the evidence behind the assertion
Only one impact is defined as a "common" impact. However, impacts are relevant for other areas of genetic testing including pharmacogenomics and somatics and more impact types will be defined there.
The "Inherited Disease" impact indicates the likelihood of inheritance (valueCodeableConcept) of a particular disease (the associated-phenotype) as well as how inheritance is likely to occur (mode-of-inheritance).
1.2.6 Other content

The profiles describing the detailed observations within a genetic report are found in the other sections of this implementation guide based on what type of testing and reporting is being done:
- The Sequenced Variants section deals with all types of variants detected by formal sequencing, including simple/discrete variants, structural variants and complex variants detected by direct sequencing, shot-gun-based sequencing and array-based testing for specific variants.
- The Cytogenomic Reporting section deals with all types of cytogenetic observations including single-code cytogenetic notations (i.e. ISCN codes), FISH test results and microarray results.
- The Pharmacogenomic Reporting section deals with genetic testing related to medication results. It primarily focuses on medication-related impacts of variation, haplotype and genotype observations.
- The Somatic Genomic Reporting section deals with non-germline variations, particularly those related to tumors and genetic-based impacts on outcomes and the effectiveness of medications and other interventions.
- The Genomic Profiling for Transplantation section deals with information related to variations relevant to matching of donor candidates with recipients, including HLA typing.
Many genetic reports will draw on more than one of these areas. For example, a somatic report will typically include sequencing information as well as information on likely tumor susceptibility to particular medications. Reports should draw on whatever sections are relevant.










