This page is part of the Pharmaceutical Quality (Industry) (v1.0.0: STU1) based on FHIR (HL7® FHIR® Standard) v5.0.0. This is the current published version. For a full list of available versions, see the Directory of published versions
Details about primary and secondary packaging components for drug substance and drug product.
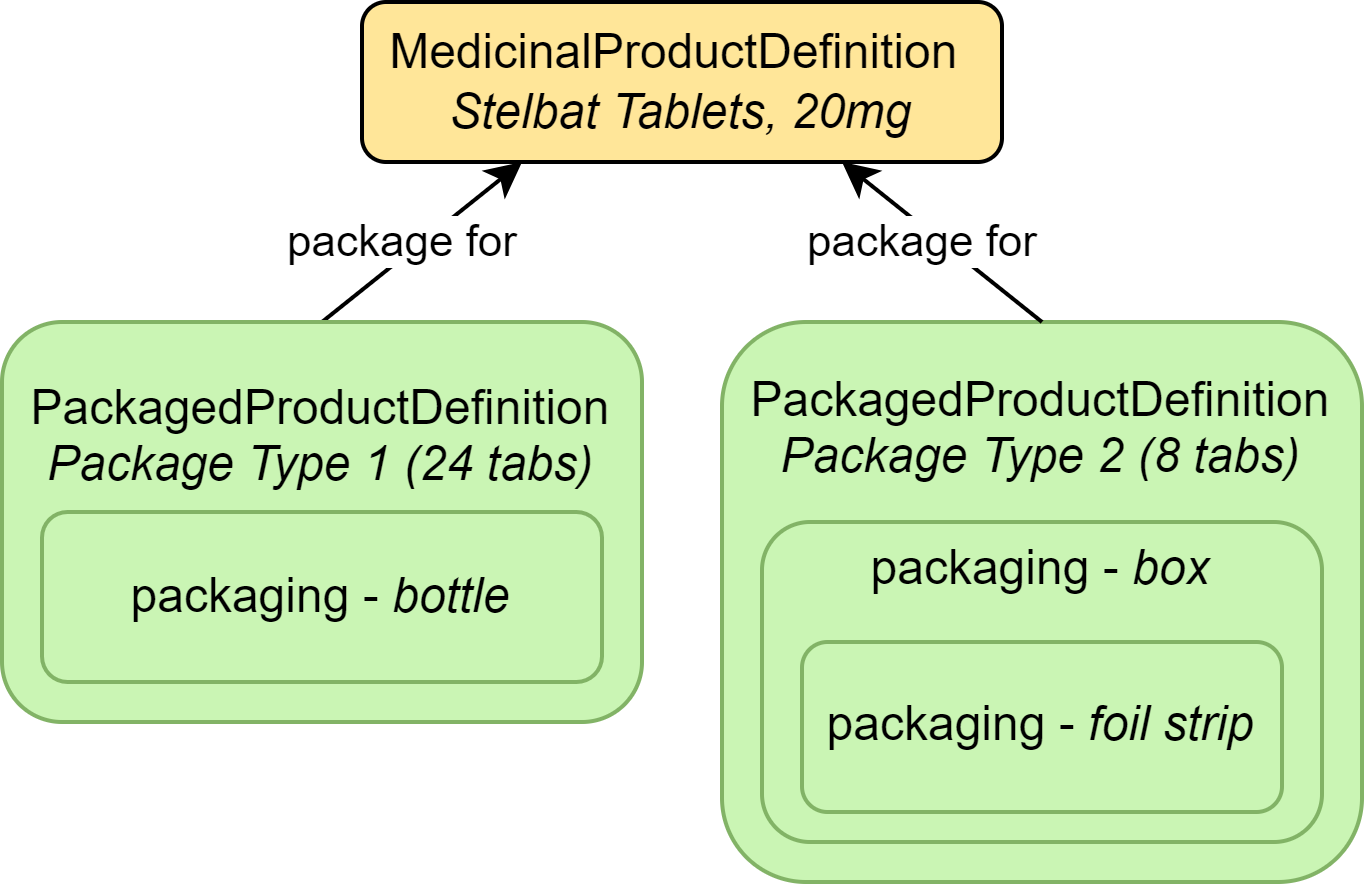 |
| MedicinalProductDefinition | The drug product (Stelbat tablets, 20mg) |
| PackagedProductDefinition | Information about the packaging for the drug product |
CTD section synthetic source data samples (PDF):
XML and JSON examples of synthetic quality data:
HTML presentation example of synthetic quality data:
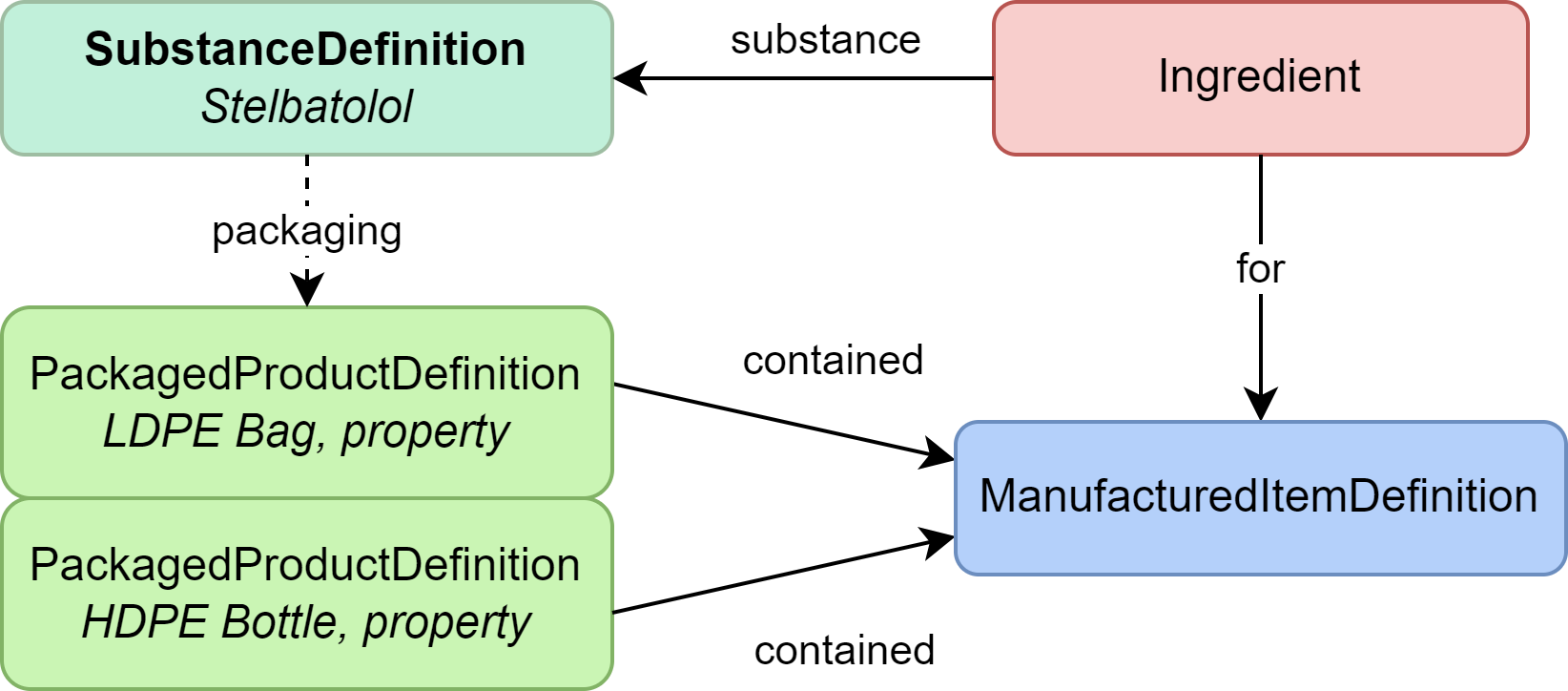 |
| Substance Definition | Definition of this drug substance itself (Stelbatolol) |
| Ingredient | Connection to the substance as something manufactured (used here mainly for linkage) |
| ManufacturedItemDefinition | The substance as a physical type of substance that can be packaged (used here mainly for linkage) |
| PackagedProductDefinition | Information about the packaging for the drug substance |
CTD section synthetic source data samples (PDF):
XML and JSON examples of synthetic quality data:
HTML presentation example of synthetic quality data: