This page is part of the Genetic Reporting Implementation Guide (v1.0.0: STU 1) based on FHIR R4. The current version which supercedes this version is 2.0.0. For a full list of available versions, see the Directory of published versions 
The content in this section may be significantly revised prior to the next ballot after initial use and feedback.
This section provides guidance for genomic reporting about the implication of a patient's or tumor's genetics on the behavior of one or more medications. This includes making recommendations for medication adjustments. This portion of the implementation guide relies on the content in the General Genomic Reporting and Variant Reporting portions of this implementation guide. Pharmacogenomic reports supplement this information with a set of pharmacogenomic-specific implication profiles. Implementers of pharmacogenomic reporting may also be interested in the Somatic Reporting section of this implementation guide as it includes several profiles dealing with the implication of medications on cancers.
While not specifically profiled in this version of the IG, some additional constraints will typically apply to somatic profiles. Patient will usually be present, however pharmacogenomics can theoretically be relevant for environmental samples (e.g. to determine the most effective way to deal with a persistent environmental pathogen).
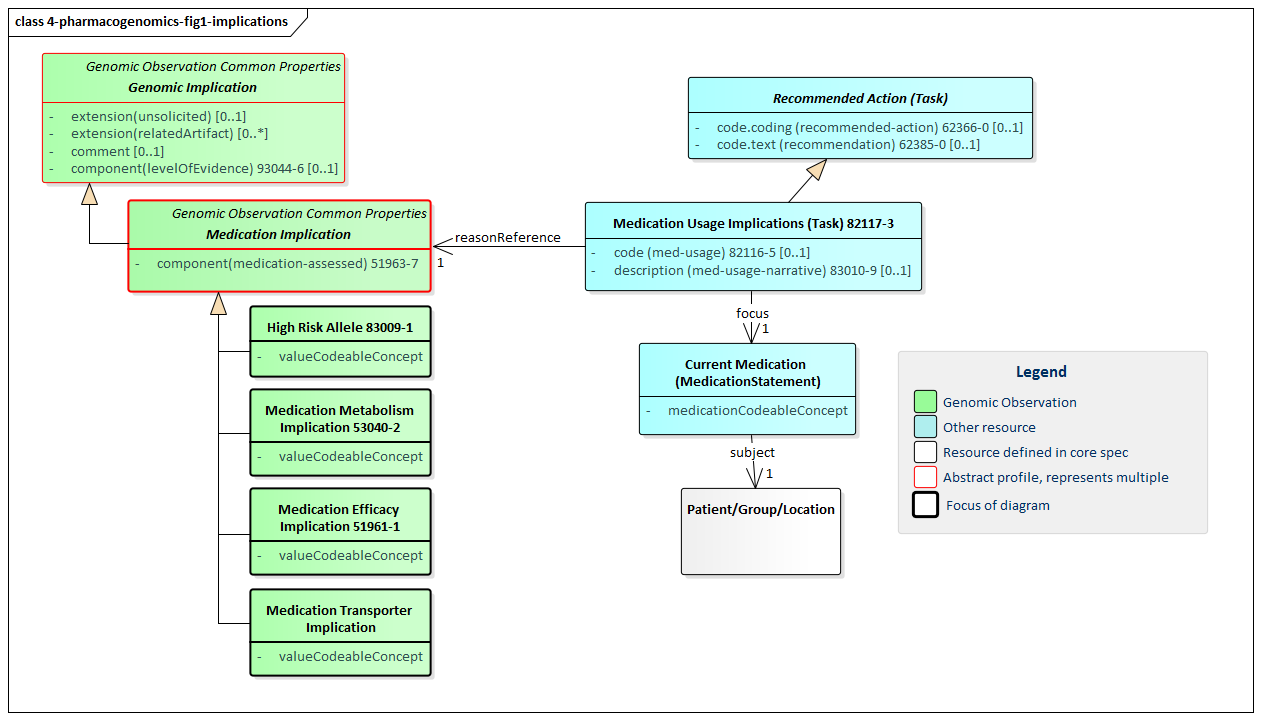
Figure 1: Pharmacogenomic Implications
(Profile links: Genetic Implication, Medication Implication, High Risk Allele, Medication Metabolism Implication, Medication Transporter Implication, Medication Efficacy Implication )
All pharmacogenomic implication profiles inherit from the abstract Genetic Implication profile. They also inherit from a common abstract Medication Implication profile which includes the mandatory code that identifies the medication whose implication is being described. Because this is an international profile, no guidance is provided on drug coding systems. The typical or FHIR-mandated jurisdictional code system(s) should be used.
There are three types of implications defined in this profile:
These implications can all be associated with the Medication Usage Implication profile which allows making a recommendation for the patient's medication therapy (e.g. discontinuing a medication, altering dosage, etc.).
The modeling of the usage implications may also change if the modeling of implications change as discussed in the General Genomic Reporting section.