This page is part of the Making EHR Data MOre available for Research and Public Health (MedMorph) Research Content IG (v0.1.0: STU 1 Ballot 1) based on FHIR (HL7® FHIR® Standard) R4. No current official version has been published yet. For a full list of available versions, see the Directory of published versions
This section defines the specific requirements for systems wishing to conform to actors specified in this MedMorph Research Content IG. The specification focuses on using the Backend Service App to perform the ETL process used for data partner on boarding within research networks.
Before reading this formal specification, implementers should first be familiar with these sections of the specification:
This implementation guide uses specific terminology to flag statements that have relevance for the evaluation of conformance with the guide:
SHALL indicates requirements that must be met to be conformant with the specification.
SHOULD indicates behaviors that are strongly recommended (and which may result in interoperability issues or sub-optimal behavior if not adhered to), but which do not, for this version of the specification, affect the determination of specification conformance.
MAY describes optional behaviors that are free to consider but where the is no recommendation for or against adoption.
Actors and Systems asserting conformance to this implementation guide have to implement the requirements outlined in the corresponding capability statements. The following definition of MUST SUPPORT is to be used in the implementation of the requirements.
This specification makes significant use of FHIR profiles, search parameter definitions, and terminology artifacts to describe the content to be shared as part of MedMorph Research Data Exchange Content IG workflows. The implementation guide is based on FHIR R4 and profiles are listed for each interaction.
The full set of profiles defined in this implementation guide can be found by following the links on the MedMorph FHIR Artifacts page.
This IG leverages the MedMorph RA IG defined by HL7 Public Health WG as the reference architecture for automation and implementing the research data exchange use case.
This IG leverages the CDMH IG profiles to export and map data that can be transformed to PCORnet CDM, i2b2, OMOP and Sentinel data models. The CDMH profiles provide a complete set of mapping from FHIR profiles to PCORnet CDM which will be leveraged by the implementers of this IG to transform the FHIR resources to the appropriate CDM hosted by the Data Mart.
This IG leverages the US Core set of profiles defined by HL7 for sharing non-veterinary EMR individual health data in the U.S. Where US Core profiles exist, this IG either leverages them directly or uses them as a base for any additional constraints needed to support the research use cases. If no constraints are needed, this IG does not define any profiles.
This IG leverages the BulkData Access IG defined by HL7 Infrastructure WG for
* Performing a Bulk Export of data from an EHR that can be used to populate the Data Mart CDM.
* Enabling authentication and authorization between various actors involved in the workflows.
This section describes the requirements of the Research Knowledge Artifact to implement the outlined use cases. In order to define the Knowledge Artifact the following workflow is used.
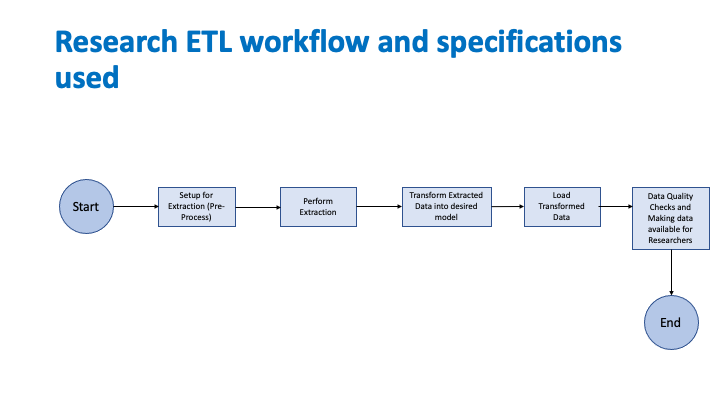
The Knowledge Artifact should have appropriate actions for successful data extraction from an EHR, transform the data to appropriate CDM and then load the data into the Data Mart CDM. However the MedMorph RA IG currently defines actions only to extract the data from the EHR but does not provide generic actions for transforming and loading the data into the data mart. So the Knowledge Artifact for the Research Data Exchange will contain the actions to extract the data but not the actions for transformation and loading the data mart.
This section outlines how the SMART on FHIR Backend Services Authorization will be used by the MedMorph Research Data Exchange Content implementation guide.
The system actors namely EHRs and Backend Service App are required to use the SMART on FHIR Backend Services Authorization mechanisms as outlined below for the following interactions
System actors acting as servers (EHRs) SHALL advertise conformance to SMART Backend Services by hosting a Well-Known Uniform Resource Identifiers (URIs) as defined in the Bulk Data Access IG Authorization Section specification.
System actors acting as servers SHALL include token_endpoint, scopes_supported, token_endpoint_auth_methods_supported and token_endpoint_auth_signing_alg_values_supported as defined in the Bulk Data Access IG Authorization Section specification.
When System actors act as clients (Backend Service App), they SHALL share their JSON Web Key Set (JWKS) with the server System actors (EHRs and NCHS data stores) using Uniform Resource Locators (URLs) as defined in the Bulk Data Access IG Authorization Section specification.
System actors acting as clients SHALL obtain the access token as defined in the Bulk Data Access IG Authorization Section specification.
For the research data exchange use cases, EHRs SHALL support the system/*.read scopes.
The healthcare organization’s existing processes along with the EHRs authorization server SHALL verify consent and other policy requirements before allowing the Backend Service App to access the data to be extracted for research purposes. The processes to be verified by the health care organizations include any consent required from patients, IRB approvals and any other agreements required based on the health care organization policies.
EHRs SHALL support the creation of Group resource to identify the patients who have consented to be participating in a research program.
EHRs SHALL associate Groups with specific clients who can query the Group for data.
EHRs SHALL allow clients to retrieve Group resource by name. When multiple Group resources meet the criteria, the EHR SHOULD return each of the Group resources that match the name, unless the EHR can narrow down the Group resource to only one.
EHRs SHALL allow clients to retrieve Group resource by identifier. When multiple Group resources meet the criteria, the EHR SHOULD return each of the Group resources that match the name, unless the EHR can narrow down the Group resource to only one.
EHRs and the Backend Service App SHOULD pre-coordinate the group names and/or identifiers that can be used for retrieval of data.
EHRs SHALL support the Bulk Data Access Group export operation to export the data required for the data mart population.
EHRs SHALL support the Group export operation parameters for all Group export operations.
EHRs SHALL support the US Core Profiles as outlined in the EHR Capability Statement for the Backend Service App to access patient data using the Group export operation.
EHRs SHOULD support the CDMH IG Profiles for the Backend Service App to access patient data using the Group export operation for populating multiple common data models.
The BSA SHALL allow the healthcare organization to activate/deactivate a specific Knowledge Artifact. Activation indicates applying the Knowledge Artifact and deactivation indicates not applying the Knowledge Artifact for events occurring within the healthcare organization.
The BSA SHALL implement FhirPath expression processing to process the MedMoprh Research Data Exchange Knowledge Artifact actions.
The BSA SHALL schedule jobs to perform data extraction from EHRs at scheduled time intervals per the Knowledge Artifact.
The BSA acting as a client SHALL use the US Core Server APIs as outlined in the EHR Capability Statement to access patient data from the EHR
The BSA acting as a client SHOULD use the CDMH IG Profiles to extract data required to populate the common data models.
ndjson to appropriate data models and load the data models following the CDMH IG Mappings from FHIR to CDMs.