This page is part of the FHIR Specification (v3.3.0: R4 Ballot 2). The current version which supercedes this version is 5.0.0. For a full list of available versions, see the Directory of published versions  . Page versions: R5 R4B R4 R3 R2
. Page versions: R5 R4B R4 R3 R2
Pharmacy  Work Group Work Group | Maturity Level: 3 | Trial Use | Compartments: Not linked to any defined compartments |
This resource is primarily used for the identification and definition of a medication for the purposes of prescribing, dispensing, and administering a medication as well as for making statements about medication use.
Representing medications in the majority of healthcare settings is a matter of identifying an item from a list and then conveying a reference for the item selected either into a patient-related resource or to other applications. Additional information about the medication is frequently provided for human verification, but a full representation of the details of composition and efficacy of the medicine is conveyed by referring to drug dictionaries by means of the codes they define. There are some occasions where it is necessary to identify slightly more detail, such as when dispensing a package containing a particular medication requires identification both of the medicine and the package at once. There are also some occasions (e.g. custom formulations) where the composition of a medicine must be represented. In these cases the ingredients of the medicine have to be specified together with the amount contained, though the Medication resource does not provide full details.
The Medication resource allows for medications to be characterized by the form of the drug and the ingredient (or ingredients), as well as how it is packaged. The medication will include the ingredient(s) and their strength(s) and the package can include the amount (for example, number of tablets, volume, etc.) that is contained in a particular container (for example, 100 capsules of Amoxicillin 500mg per bottle).
The Medication resource can be used to describe a compounded (aka extemporaneous or magistral) product that is manufactured by the pharmacy at the time of dispensing. In this case there will be multiple ingredients which are typically base chemicals (for example, hydrocortisone powder) and there may be other ingredients that are manufactured products (for example, Glaxal Base).
When a medication includes a package, further details about the composition can be provided. A package has a container (vacuum packed box, jar, etc.) and a list of the products or other packages that are in the package.
This resource is referenced by ActivityDefinition, AdverseEvent, CarePlan, EntryDefinition, Flag, Group, MedicationAdministration, MedicationDispense, MedicationRequest, MedicationStatement, MedicinalProductClinicals, Procedure, SupplyDelivery and SupplyRequest
Structure
| Name | Flags | Card. | Type | Description & Constraints |
|---|---|---|---|---|
 | TU | DomainResource | Definition of a Medication Elements defined in Ancestors: id, meta, implicitRules, language, text, contained, extension, modifierExtension | |
  | Σ | 0..1 | CodeableConcept | Codes that identify this medication SNOMED CT Medication Codes (Example) |
  | Σ | 0..1 | code | active | inactive | entered-in-error MedicationStatus (Required) |
  | Σ | 0..1 | Reference(Organization) | Manufacturer of the item |
  | 0..1 | CodeableConcept | powder | tablets | capsule + SNOMED CT Form Codes (Example) | |
  | ?!Σ | 0..1 | SimpleQuantity | Amount of drug in package |
  | 0..* | BackboneElement | Active or inactive ingredient | |
   | 1..1 | The product contained | ||
    | CodeableConcept | |||
   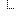 | Reference(Substance | Medication) | |||
   | 0..1 | boolean | Active ingredient indicator | |
  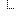 | 0..1 | Ratio | Quantity of ingredient present | |
 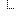 | 0..1 | BackboneElement | Details about packaged medications | |
   | 0..1 | string | Identifier assigned to batch | |
   | 0..1 | dateTime | When batch will expire | |
  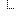 | 0..1 | string | Identifier assigned to a drug at the time of manufacture | |
 Documentation for this format Documentation for this format | ||||
UML Diagram (Legend)
XML Template
<Medication xmlns="http://hl7.org/fhir"><!-- from Resource: id, meta, implicitRules, and language --> <!-- from DomainResource: text, contained, extension, and modifierExtension --> <code><!-- 0..1 CodeableConcept Codes that identify this medication --></code> <status value="[code]"/><!-- 0..1 active | inactive | entered-in-error --> <manufacturer><!-- 0..1 Reference(Organization) Manufacturer of the item --></manufacturer> <form><!-- 0..1 CodeableConcept powder | tablets | capsule + --></form> <amount><!-- 0..1 Quantity(SimpleQuantity) Amount of drug in package --></amount> <ingredient> <!-- 0..* Active or inactive ingredient --> <item[x]><!-- 1..1 CodeableConcept|Reference(Substance|Medication) The product contained --></item[x]> <isActive value="[boolean]"/><!-- 0..1 Active ingredient indicator --> <amount><!-- 0..1 Ratio Quantity of ingredient present --></amount> </ingredient> <batch> <!-- 0..1 Details about packaged medications --> <lotNumber value="[string]"/><!-- 0..1 Identifier assigned to batch --> <expirationDate value="[dateTime]"/><!-- 0..1 When batch will expire --> <serialNumber value="[string]"/><!-- 0..1 Identifier assigned to a drug at the time of manufacture --> </batch> </Medication>
JSON Template
{ "resourceType" : "Medication",
// from Resource: id, meta, implicitRules, and language
// from DomainResource: text, contained, extension, and modifierExtension
"code" : { CodeableConcept }, // Codes that identify this medication
"status" : "<code>", // active | inactive | entered-in-error
"manufacturer" : { Reference(Organization) }, // Manufacturer of the item
"form" : { CodeableConcept }, // powder | tablets | capsule +
"amount" : { Quantity(SimpleQuantity) }, // Amount of drug in package
"ingredient" : [{ // Active or inactive ingredient
// item[x]: The product contained. One of these 2:
"itemCodeableConcept" : { CodeableConcept },
"itemReference" : { Reference(Substance|Medication) },
"isActive" : <boolean>, // Active ingredient indicator
"amount" : { Ratio } // Quantity of ingredient present
}],
"batch" : { // Details about packaged medications
"lotNumber" : "<string>", // Identifier assigned to batch
"expirationDate" : "<dateTime>", // When batch will expire
"serialNumber" : "<string>" // Identifier assigned to a drug at the time of manufacture
}
}
"resourceType" : "Medication",
// from Resource: id, meta, implicitRules, and language
// from DomainResource: text, contained, extension, and modifierExtension
"code" : { CodeableConcept }, // Codes that identify this medication
"status" : "<code>", // active | inactive | entered-in-error
"manufacturer" : { Reference(Organization) }, // Manufacturer of the item
"form" : { CodeableConcept }, // powder | tablets | capsule +
"amount" : { Quantity(SimpleQuantity) }, // Amount of drug in package
"ingredient" : [{ // Active or inactive ingredient
// item[x]: The product contained. One of these 2:
"itemCodeableConcept" : { CodeableConcept },
"itemReference" : { Reference(Substance|Medication) },
"isActive" : <boolean>, // Active ingredient indicator
"amount" : { Ratio } // Quantity of ingredient present
}],
"batch" : { // Details about packaged medications
"lotNumber" : "<string>", // Identifier assigned to batch
"expirationDate" : "<dateTime>", // When batch will expire
"serialNumber" : "<string>" // Identifier assigned to a drug at the time of manufacture
}
}
Turtle Template
@prefix fhir: <http://hl7.org/fhir/> .[ a fhir:Medication; fhir:nodeRole fhir:treeRoot; # if this is the parser root # from Resource: .id, .meta, .implicitRules, and .language # from DomainResource: .text, .contained, .extension, and .modifierExtension fhir:Medication.code [ CodeableConcept ]; # 0..1 Codes that identify this medication fhir:Medication.status [ code ]; # 0..1 active | inactive | entered-in-error fhir:Medication.manufacturer [ Reference(Organization) ]; # 0..1 Manufacturer of the item fhir:Medication.form [ CodeableConcept ]; # 0..1 powder | tablets | capsule + fhir:Medication.amount [ Quantity(SimpleQuantity) ]; # 0..1 Amount of drug in package fhir:Medication.ingredient [ # 0..* Active or inactive ingredient # Medication.ingredient.item[x] : 1..1 The product contained. One of these 2 fhir:Medication.ingredient.itemCodeableConcept [ CodeableConcept ] fhir:Medication.ingredient.itemReference [ Reference(Substance|Medication) ] fhir:Medication.ingredient.isActive [ boolean ]; # 0..1 Active ingredient indicator fhir:Medication.ingredient.amount [ Ratio ]; # 0..1 Quantity of ingredient present ], ...; fhir:Medication.batch [ # 0..1 Details about packaged medications fhir:Medication.batch.lotNumber [ string ]; # 0..1 Identifier assigned to batch fhir:Medication.batch.expirationDate [ dateTime ]; # 0..1 When batch will expire fhir:Medication.batch.serialNumber [ string ]; # 0..1 Identifier assigned to a drug at the time of manufacture ]; ]
Changes since R3
| Medication | |
| Medication.amount |
|
| Medication.ingredient.item[x] |
|
| Medication.batch |
|
| Medication.batch.lotNumber |
|
| Medication.batch.expirationDate |
|
| Medication.batch.serialNumber |
|
| Medication.isBrand |
|
| Medication.isOverTheCounter |
|
| Medication.package |
|
| Medication.image |
|
See the Full Difference for further information
This analysis is available as XML or JSON.
See R2 <--> R3 Conversion Maps (status = 23 tests that all execute ok. 16 fail round-trip testing and 1 r3 resources are invalid (1 errors).). Note: these have note yet been updated to be R3 to R4
Structure
| Name | Flags | Card. | Type | Description & Constraints |
|---|---|---|---|---|
 | TU | DomainResource | Definition of a Medication Elements defined in Ancestors: id, meta, implicitRules, language, text, contained, extension, modifierExtension | |
  | Σ | 0..1 | CodeableConcept | Codes that identify this medication SNOMED CT Medication Codes (Example) |
  | Σ | 0..1 | code | active | inactive | entered-in-error MedicationStatus (Required) |
  | Σ | 0..1 | Reference(Organization) | Manufacturer of the item |
  | 0..1 | CodeableConcept | powder | tablets | capsule + SNOMED CT Form Codes (Example) | |
  | ?!Σ | 0..1 | SimpleQuantity | Amount of drug in package |
  | 0..* | BackboneElement | Active or inactive ingredient | |
   | 1..1 | The product contained | ||
    | CodeableConcept | |||
    | Reference(Substance | Medication) | |||
   | 0..1 | boolean | Active ingredient indicator | |
   | 0..1 | Ratio | Quantity of ingredient present | |
  | 0..1 | BackboneElement | Details about packaged medications | |
   | 0..1 | string | Identifier assigned to batch | |
   | 0..1 | dateTime | When batch will expire | |
   | 0..1 | string | Identifier assigned to a drug at the time of manufacture | |
 Documentation for this format Documentation for this format | ||||
XML Template
<Medication xmlns="http://hl7.org/fhir"><!-- from Resource: id, meta, implicitRules, and language --> <!-- from DomainResource: text, contained, extension, and modifierExtension --> <code><!-- 0..1 CodeableConcept Codes that identify this medication --></code> <status value="[code]"/><!-- 0..1 active | inactive | entered-in-error --> <manufacturer><!-- 0..1 Reference(Organization) Manufacturer of the item --></manufacturer> <form><!-- 0..1 CodeableConcept powder | tablets | capsule + --></form> <amount><!-- 0..1 Quantity(SimpleQuantity) Amount of drug in package --></amount> <ingredient> <!-- 0..* Active or inactive ingredient --> <item[x]><!-- 1..1 CodeableConcept|Reference(Substance|Medication) The product contained --></item[x]> <isActive value="[boolean]"/><!-- 0..1 Active ingredient indicator --> <amount><!-- 0..1 Ratio Quantity of ingredient present --></amount> </ingredient> <batch> <!-- 0..1 Details about packaged medications --> <lotNumber value="[string]"/><!-- 0..1 Identifier assigned to batch --> <expirationDate value="[dateTime]"/><!-- 0..1 When batch will expire --> <serialNumber value="[string]"/><!-- 0..1 Identifier assigned to a drug at the time of manufacture --> </batch> </Medication>
JSON Template
{ "resourceType" : "Medication",
// from Resource: id, meta, implicitRules, and language
// from DomainResource: text, contained, extension, and modifierExtension
"code" : { CodeableConcept }, // Codes that identify this medication
"status" : "<code>", // active | inactive | entered-in-error
"manufacturer" : { Reference(Organization) }, // Manufacturer of the item
"form" : { CodeableConcept }, // powder | tablets | capsule +
"amount" : { Quantity(SimpleQuantity) }, // Amount of drug in package
"ingredient" : [{ // Active or inactive ingredient
// item[x]: The product contained. One of these 2:
"itemCodeableConcept" : { CodeableConcept },
"itemReference" : { Reference(Substance|Medication) },
"isActive" : <boolean>, // Active ingredient indicator
"amount" : { Ratio } // Quantity of ingredient present
}],
"batch" : { // Details about packaged medications
"lotNumber" : "<string>", // Identifier assigned to batch
"expirationDate" : "<dateTime>", // When batch will expire
"serialNumber" : "<string>" // Identifier assigned to a drug at the time of manufacture
}
}
"resourceType" : "Medication",
// from Resource: id, meta, implicitRules, and language
// from DomainResource: text, contained, extension, and modifierExtension
"code" : { CodeableConcept }, // Codes that identify this medication
"status" : "<code>", // active | inactive | entered-in-error
"manufacturer" : { Reference(Organization) }, // Manufacturer of the item
"form" : { CodeableConcept }, // powder | tablets | capsule +
"amount" : { Quantity(SimpleQuantity) }, // Amount of drug in package
"ingredient" : [{ // Active or inactive ingredient
// item[x]: The product contained. One of these 2:
"itemCodeableConcept" : { CodeableConcept },
"itemReference" : { Reference(Substance|Medication) },
"isActive" : <boolean>, // Active ingredient indicator
"amount" : { Ratio } // Quantity of ingredient present
}],
"batch" : { // Details about packaged medications
"lotNumber" : "<string>", // Identifier assigned to batch
"expirationDate" : "<dateTime>", // When batch will expire
"serialNumber" : "<string>" // Identifier assigned to a drug at the time of manufacture
}
}
Turtle Template
@prefix fhir: <http://hl7.org/fhir/> .[ a fhir:Medication; fhir:nodeRole fhir:treeRoot; # if this is the parser root # from Resource: .id, .meta, .implicitRules, and .language # from DomainResource: .text, .contained, .extension, and .modifierExtension fhir:Medication.code [ CodeableConcept ]; # 0..1 Codes that identify this medication fhir:Medication.status [ code ]; # 0..1 active | inactive | entered-in-error fhir:Medication.manufacturer [ Reference(Organization) ]; # 0..1 Manufacturer of the item fhir:Medication.form [ CodeableConcept ]; # 0..1 powder | tablets | capsule + fhir:Medication.amount [ Quantity(SimpleQuantity) ]; # 0..1 Amount of drug in package fhir:Medication.ingredient [ # 0..* Active or inactive ingredient # Medication.ingredient.item[x] : 1..1 The product contained. One of these 2 fhir:Medication.ingredient.itemCodeableConcept [ CodeableConcept ] fhir:Medication.ingredient.itemReference [ Reference(Substance|Medication) ] fhir:Medication.ingredient.isActive [ boolean ]; # 0..1 Active ingredient indicator fhir:Medication.ingredient.amount [ Ratio ]; # 0..1 Quantity of ingredient present ], ...; fhir:Medication.batch [ # 0..1 Details about packaged medications fhir:Medication.batch.lotNumber [ string ]; # 0..1 Identifier assigned to batch fhir:Medication.batch.expirationDate [ dateTime ]; # 0..1 When batch will expire fhir:Medication.batch.serialNumber [ string ]; # 0..1 Identifier assigned to a drug at the time of manufacture ]; ]
Changes since DSTU2
| Medication | |
| Medication.amount |
|
| Medication.ingredient.item[x] |
|
| Medication.batch |
|
| Medication.batch.lotNumber |
|
| Medication.batch.expirationDate |
|
| Medication.batch.serialNumber |
|
| Medication.isBrand |
|
| Medication.isOverTheCounter |
|
| Medication.package |
|
| Medication.image |
|
See the Full Difference for further information
This analysis is available as XML or JSON.
See R2 <--> R3 Conversion Maps (status = 23 tests that all execute ok. 16 fail round-trip testing and 1 r3 resources are invalid (1 errors).). Note: these have note yet been updated to be R3 to R4
Alternate definitions: Master Definition XML + JSON, XML Schema/Schematron + JSON Schema, ShEx (for Turtle) + see the extensions & the dependency analysis
| Path | Definition | Type | Reference |
|---|---|---|---|
| Medication.code | A coded concept that defines the type of a medication | Example | SNOMED CT Medication Codes |
| Medication.status | A coded concept defining if the medication is in active use | Required | MedicationStatus |
| Medication.form | A coded concept defining the form of a medication | Example | SNOMED CT Form Codes |
Medication does not have a status. If Medication was used to support a formulary use case, then an extension can be used to convey formulary statuses, such as active (e.g. the medication can be ordered) or inactive (e.g. the medication can be documented, but not ordered). Pharmacy is evaluating formulary use cases. Feedback is encouraged to the Pharmacy working group.
Search parameters for this resource. The common parameters also apply. See Searching for more information about searching in REST, messaging, and services.
| Name | Type | Description | Expression | In Common |
| code | token | Codes that identify this medication | Medication.code | 4 Resources |
| expiration-date | date | When batch will expire | Medication.batch.expirationDate | |
| form | token | powder | tablets | capsule + | Medication.form | |
| ingredient | reference | The product contained | Medication.ingredient.item.as(Reference) (Medication, Substance) | |
| ingredient-code | token | The product contained | Medication.ingredient.item.as(CodeableConcept) | |
| lot-number | token | Identifier assigned to batch | Medication.batch.lotNumber | |
| manufacturer | reference | Manufacturer of the item | Medication.manufacturer (Organization) | |
| serial-number | token | Identifier assigned to a drug at the time of manufacture | Medication.batch.serialNumber | |
| status | token | active | inactive | entered-in-error | Medication.status |