This page is part of the Clinical Study Schedule of Activities (v1.0.0: STU1) based on FHIR R4. This is the current published version. For a full list of available versions, see the Directory of published versions 
The basic SoA visit timings (that is, the subject study day/times on which required activities should be performed) describes the optimal schedule to meet a protocols research objectives. Practical considerations in the clinic make it sensible to offer investigational sites some flexibility to accommodate issues such as visits falling on weekends, or hospital services availability (e.g. for X-Rays), or other logistical issues. These permitted variances are usually specified in SoA in the form of “visit date/time +/- duration” (V3 +/- 1 day). The flexibility around a planned visit/encounter may be symmetric or asymmetrically around a target date (e.g., no more than 2 days before and up to 4 days after the target date). The adherence of an investigational site to the target and permitted visit windows ensures compliance with the protocol timings, and may be used as a measure of ‘site quality’.
The existing FHIR structure for specifying ranges over which a related action can occur uses the Period datatype; which has lower and upper attributes defining the Range of dates/times that an encounter or activities can occur. This can be used to specify visit windows, but does not then permit a single target date to be specified within that visit window.
In Clinical Research it is often important to be more rigorous about when activities can occur; this broadly comes under the heading of Visit Windows. In conduct, it is important to be able to specify when an encounter (and the related observations) should occur, but allow some flexibility to deal with logistical issues arising (such as a public holiday, device maintenance, study participant travel arrangements). The flexibility can be asymmetrically arranged around a target date (e.g., no more than 2 days before and up to 4 days after the target date).
The adherence of an investigational site to these windows often constitutes one measure of ‘site quality’. Whilst the FHIR Period datatype is ideal for describing the Range over which planned activties or encounters may occur, it has no provision for associating it with the planned target time. (More specifically FHIR v4.3.0: R4B only permits one timing[x] element to be specified per PlanDefinition.action)
The visit window use case described above is the most commonly found in SoAs, and will describe the timing details of each visit/encounter from a defined t(zero) visit (e.g. as in the Core Model SoA example). Other timing use cases may also be specified such as “Visit-N to occur 28days from Visit-0 and no earlier than 7d after Visit-(N-1). The diagram below shows the relationship between the SoA visit descriptions, the actual times activities occur and the visit window Range. Also illustrated are the 2 principal options for calculating the visit window period: (a) from the target timepoint or (b) from the reference timepoint.
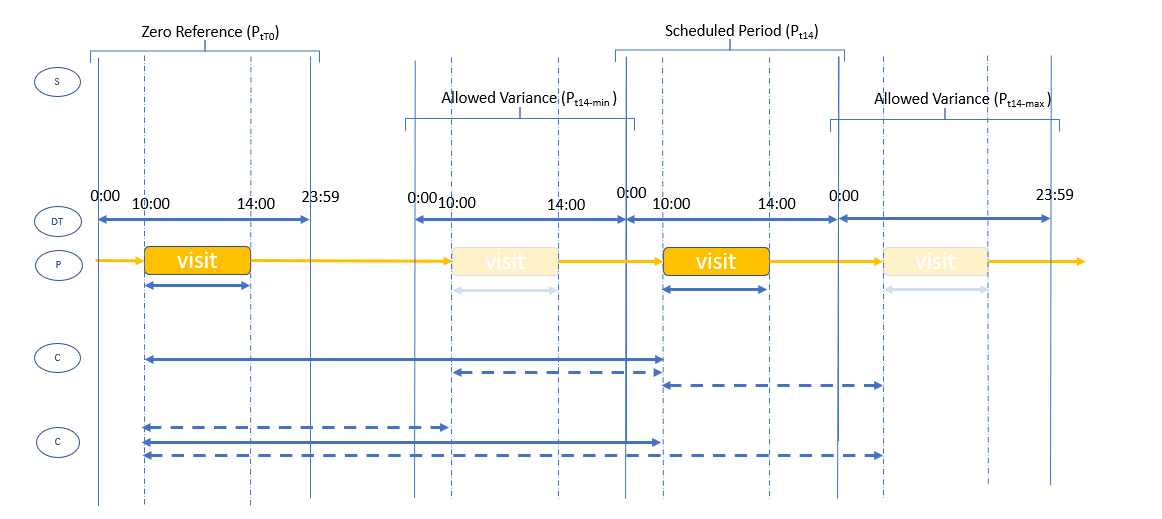
The diagram shows the relationship between an initial ‘visit’ (V1,left) and a subsequent ‘visit’ (V2, right) with permitted ‘visit window’ variances. In the example a SoA would specify this case as V2 +/ 1 day.
In order to meet the objectives above, SoA PlanDefinition ‘Visit Window’ specifications are required to support the following study types and typical general windowing use cases:
As discussed above the issue with the FHIR Definition resources (PlanDefinition and ActivityDefinition) is that they may have an offsetDuration or offsetRange and timing[x] attributes that are mutually exclusive.
An extension has been added to permit acceptableOffsetRange to the relatedAction element; using this element it is possible to define an acceptable range for an action that can be used for scheduling and reviewing.
Examples of the use of the extension can be seen in the Protocol Design Example.