This page is part of the Clinical Study Schedule of Activities (v1.0.0: STU1) based on FHIR R4. This is the current published version. For a full list of available versions, see the Directory of published versions 
In order to correctly relate data collected in a study to the times when investigation product (drug, therapy, device etc.) has been taken or used is central tenant of any study.
This requires the clear specifications of when to provide, administer and record pivotal IP events (e.g., to calculate Dose Exposure, or those procedures and outcomes associated with the patient’s exposure to the investigational product.)
The set of FHIR medication resources have been used to review how to incorporate IP administration and record keeping into FHIR specified SoA. Note: to date immunization (vaccine) and device trials have not been considered in this section (FHIR defines resources for immunization and devices separately from medications).
Investigational Product scenarios include, but are not limited to, IP:
The needs addressed as part of this use case include:
The following diagrams illustrate how the FHIR medications resources may be used to record investigational or other drug product use within an EHR. Four common administration or drug use scenarios are illustrated showing how the 5 medication resources might be used to manage and record IP use. These diagrams are designed as templates to (a) enable study SoA definitions to recognise when, where and which resources will be required in scheduling plans, and (b) guide to those resources where searches for study medication details may be found. It is expected that individual studies will select and define only those resources and relationships necessary to ensure accuracy/compliance with the protocol objectives; for example, the MedicationDispense resource might only be defined by FHIR SoAs if dispensing the IP is time-critical with respect to the administration.
In each case the details of the IP itself are considered to have a Medication resource record, with the red line(s) showing the medication.medicationReference references. All of the resources in these diagrams are not definitional resources but rather the targets for IP administration definitional resources (e.g. PlanDefinition, ActivityDefinition, etc.) that would be specified in a SoA. The blue line(s) show the relationships between the potential reporting medication resources.
The following diagrams illustrate how the FHIR medications resources may be used to record investigational or other drug product use within an EHR. Four common administration or drug use scenarios are illustrated showing how the 5 medication resources might be used to manage and record IP use. These diagrams are designed as templates to (a) enable study SoA definitions to recognise when, where and which resources will be required in scheduling plans, and (b) guide to those resources where searches for study medication details may be found. It is expected that individual studies will select and define only those resources and relationships necessary to ensure accuracy/compliance with the protocol objectives; for example, the MedicationDispense resource might only be defined by FHIR SoAs if dispensing the IP is time-critical with respect to the administration. In each case the details of the IP itself are considered to have a Medication resource record, with the red line(s) showing the medication.medicationReference references. All of the resources in these diagrams are definitional resources but rather the targets for IP administration definitional resources (e.g. PlanDefinition, ActivityDefinition, etc.) that would be specified in a SoA.
Figure Legend:
Green Circle: Start of sponsor requirement to record/review ResearchSubjectResearchSubject medication record
Red Circle: End of sponsor requirement to record/review ResearchSubject medication record
Gray Circle: eHR record recorded by study team recording a medication event - status: completed
White Circle: eHR record reflecting protocol expectation for medication intake - status: in progress, confirmed thereafter by MedicationStatement records.
Red Lines: medication.medicationReference relationships
Blue Lines: medicationXXX relationships
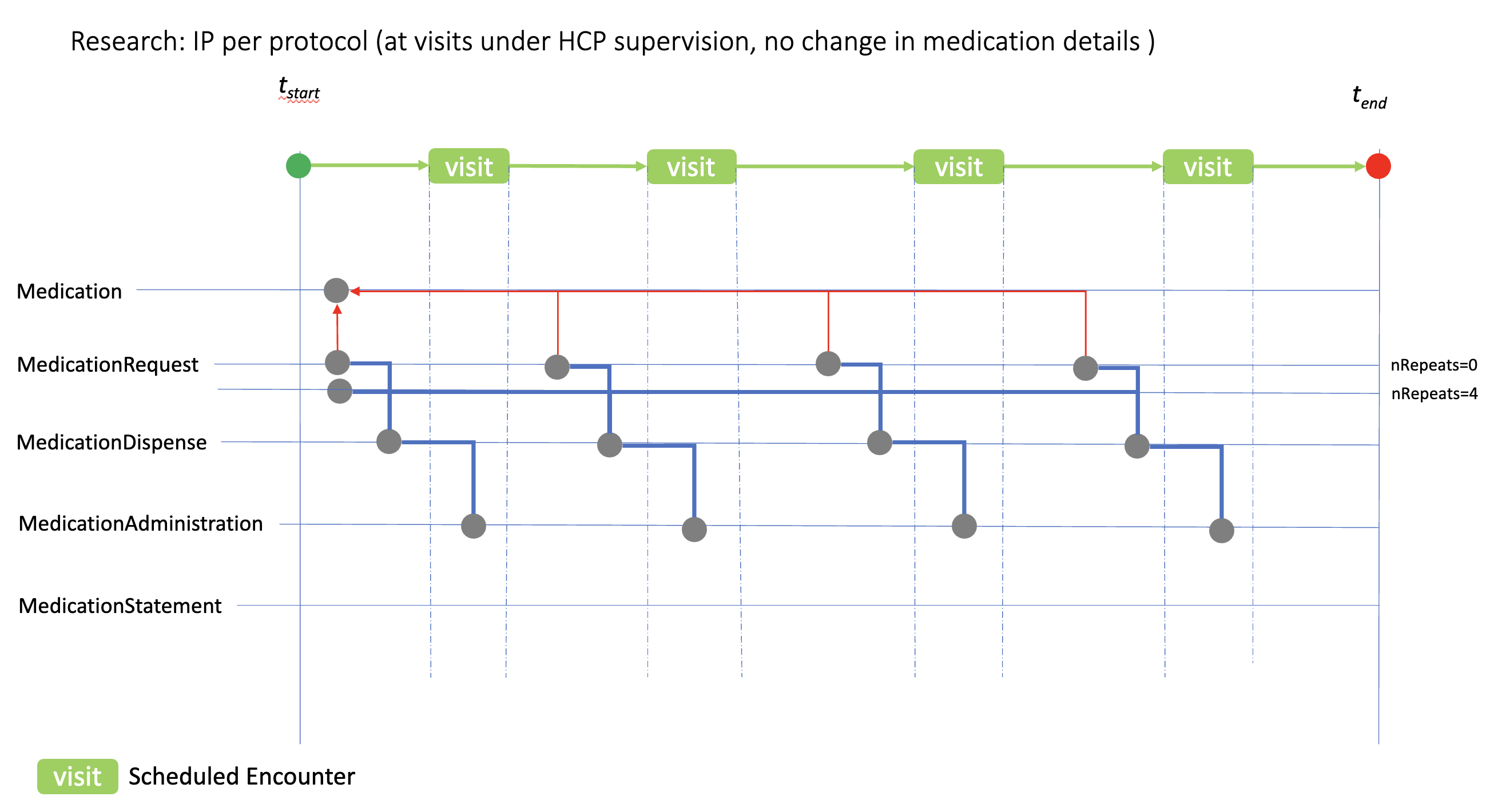
This case shows how the medication resources can be used to model ‘at visit’ IP administration, where the medication details (dose, form, etc.) remain the same at each visit. In this case two methods of using the MedicationRequest resource is shown based on the dispenseRequest.numberOfRepeatsAllowed option. The numberOfRepeatsAllowed=0 option implies individual MedicationRequest would be created prior to each visit; numberOfRepeatsAllowed=4 option implies that a single MedicationRequest is created with instructions that the IP can be dispensed 4 times.
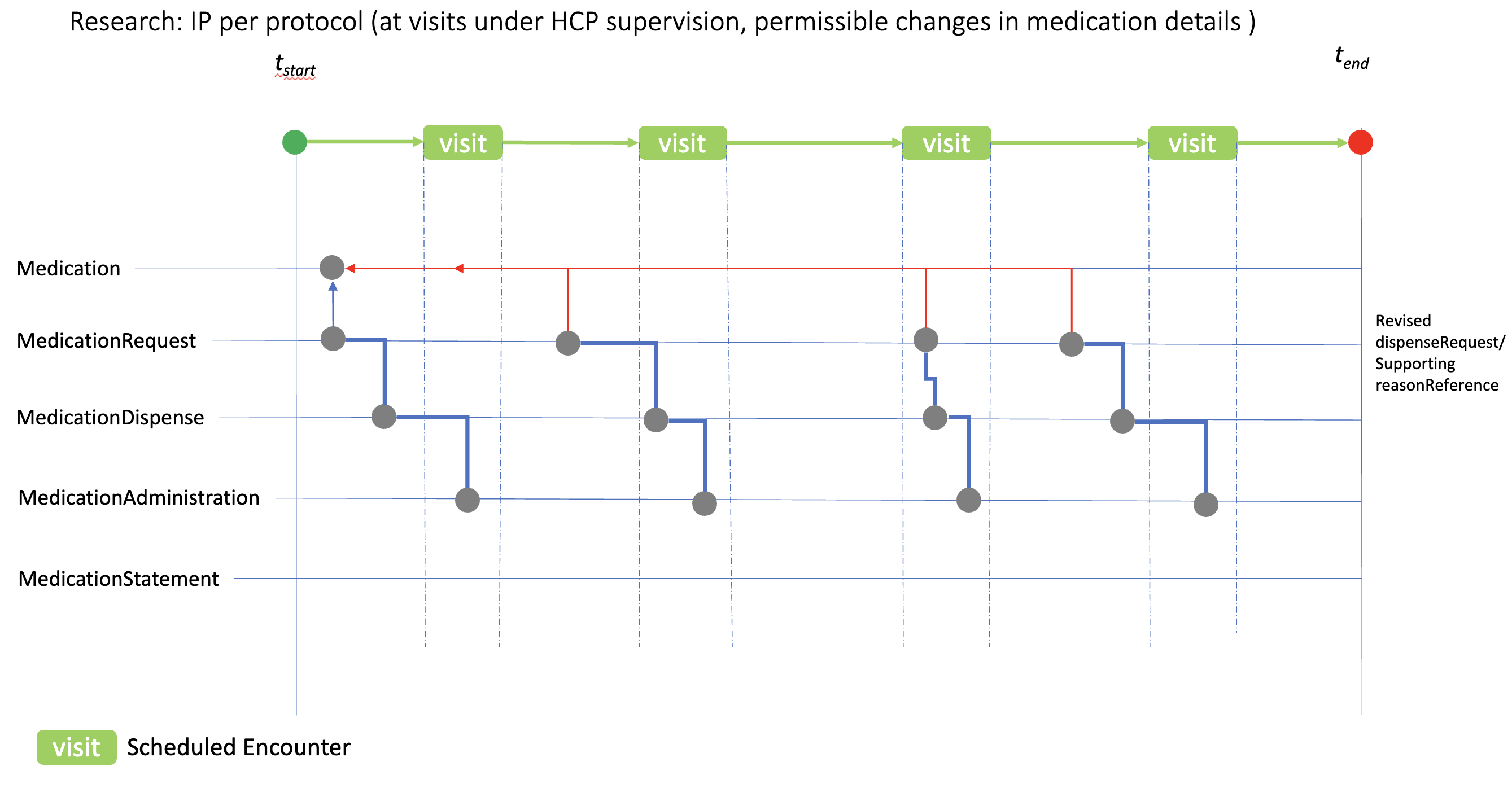
The case here illustrates the use of the medication resources when changes in IP/medication details are permitted or required by the protocol, for example dose escalation. Individual “per visit” MedicationRequest resources are used to reflect the revised IP details for dispensing, which would then be administered at the appropriate visit. reasonReference can be used to document the planned study IP changes
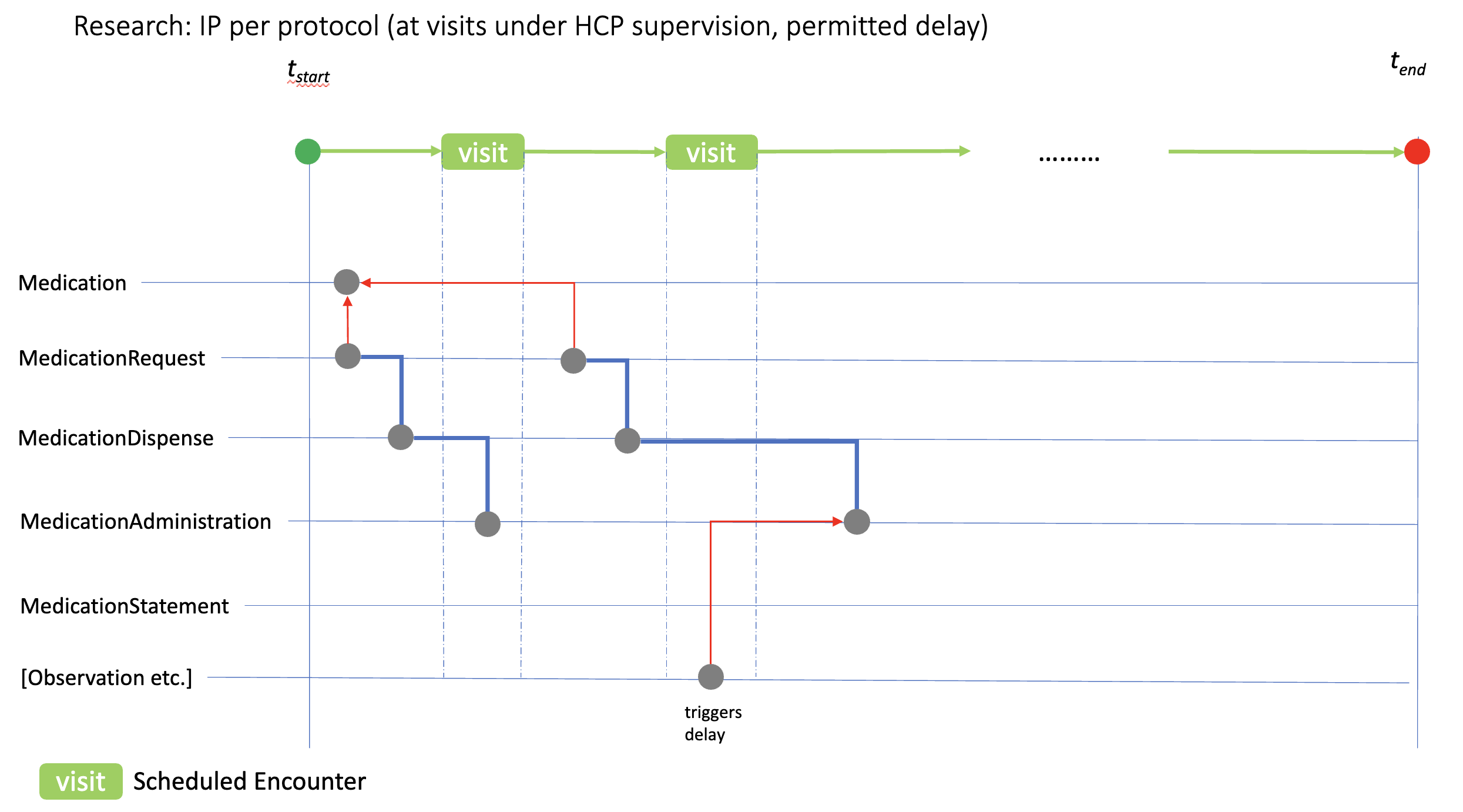
Illustrated here is the relationship between a clinical event (in this case an Observation) that requires IP administration to be delayed. If such study events are recognised in the study protocol, and are (a) critical to patient safety or (b) would compromise protocol compliance, SoA PlanDefinition.trigger relations can be used to define the rules for delaying the associated PlanDefinition.action.timing[x] that will be linked to the MedicationAdministration record. Situations that might require these revisions include out of range laboratory values, or the use of a contra-indicated medication prior to the study visit.
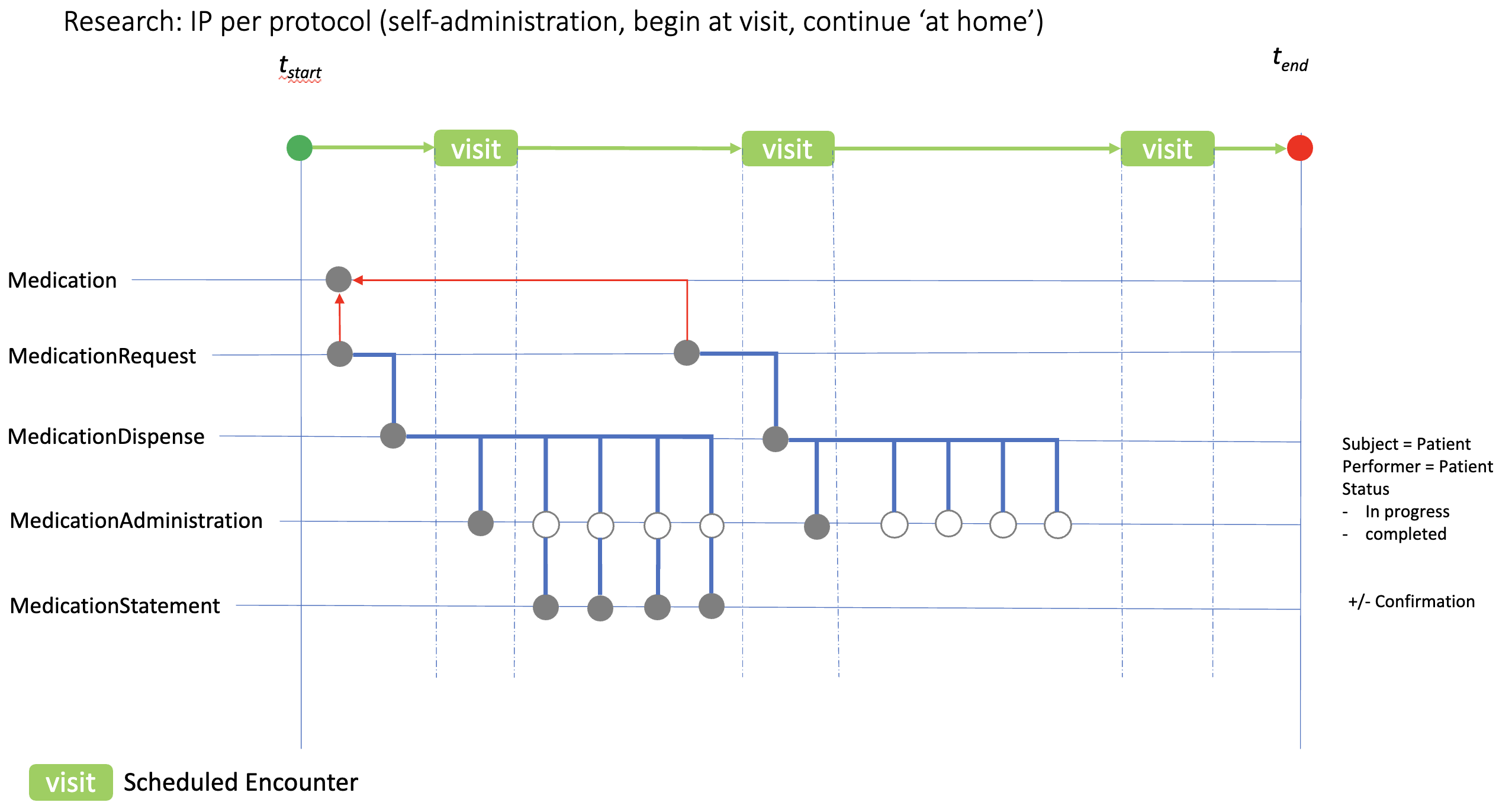
Many studies require IP to be self-administered, and to continue thereafter ‘at home’. The diagram here shows how IP self-administration might be recorded in an eHR. In this case the IP is initially taken during the clinic visit generating a MedicationAdminstration.status record of ‘completed’ (solid circle) and four ‘in progress’ records reflecting the protocol expectation. MedicationStatement resources can then be used to confirm compliance.