This page is part of the Loinc/IVD Mapping FHIR IG (v1.0.0-ballot: STU1 Ballot 1) based on FHIR (HL7® FHIR® Standard) R4. No current official version has been published yet. For a full list of available versions, see the Directory of published versions
When communicating results from a device to the Laboratory Information System (LIS), the following concepts must be mapped:
Neither the IVD Test, the LIS Test Result, nor the respective IVD Result Values are likely based on industry standard vocabulary. The manufacturer assigns analyte information such as vendor analyte code (transmission code or identifier), a name, and reference identifier, plus associated IVD Result Value codes, while the Laboratory creates LIS Test Results and its LIS Result Values for the tests they provide. Through LIS configuration tools, the IVD Test’s Vendor Analyte Code is associated with one or more LIS Test Result Codes based on context, e.g., IVD Test used with one vs. another specimen would yield a different LIS Test Results. This process has been in place for decades and has been optimized to support the Laboratory’s specific reporting requirements (including conformance to Clinical Laboratory Improvement Amendments (CLIA) for the US).
To enable analytics and clinical decision support, harmonization to a common vocabulary is critical. For laboratory test results, Logical Observation Identifiers Names and Codes (LOINC(R)) is the coding system of choice, while for non-quantitative, encoded result values, SNOMED and LOINC are both in use to enable a consistent expression. This introducing the question on how to map the LIS Test Result Code to LOINC, as well as corresponding Result Value Code to either LOINC or SNOMED, and do so consistently across all laboratories to enable analytics and clinical decision support reliably.
For various reasons, including but not limited to an LIS communicating the ordered tests to the device using LOINC, the device cannot provide the appropriate LOINC code with the test result. The LIS must provide the mapping as they associate the IVD Test and its IVD Result Value with the LIS Test Result and its LIS Result value respectively. To date this mapping process has relied on a combination of the LOINC registry, RELMA, and the individual’s knowledge of LOINC plus the LIS’ test compendium and further information on the Vendr Analyte Code’s Result Value Codes to arrive at the appropriate LOINC or SNOMED codes for the actual value where applicable.
The device manufacturer can aid in the process by providing a list of suggested LOINC codes for each of their IVD Tests, including context of the result, specimen, and other considerations that would influence the choice, as well as the appropriate LOINC or SNOMED codes for their IVD Result Values. Such guidance would help reduce the scope of potential LOINC and SNOMED codes to consider, thus improving efficiency and quality of the mapping process, particularly across laboratories, i.e., arriving at the same LOINC or SNOMED code for the same test.
The following diagram may help further clarify that:
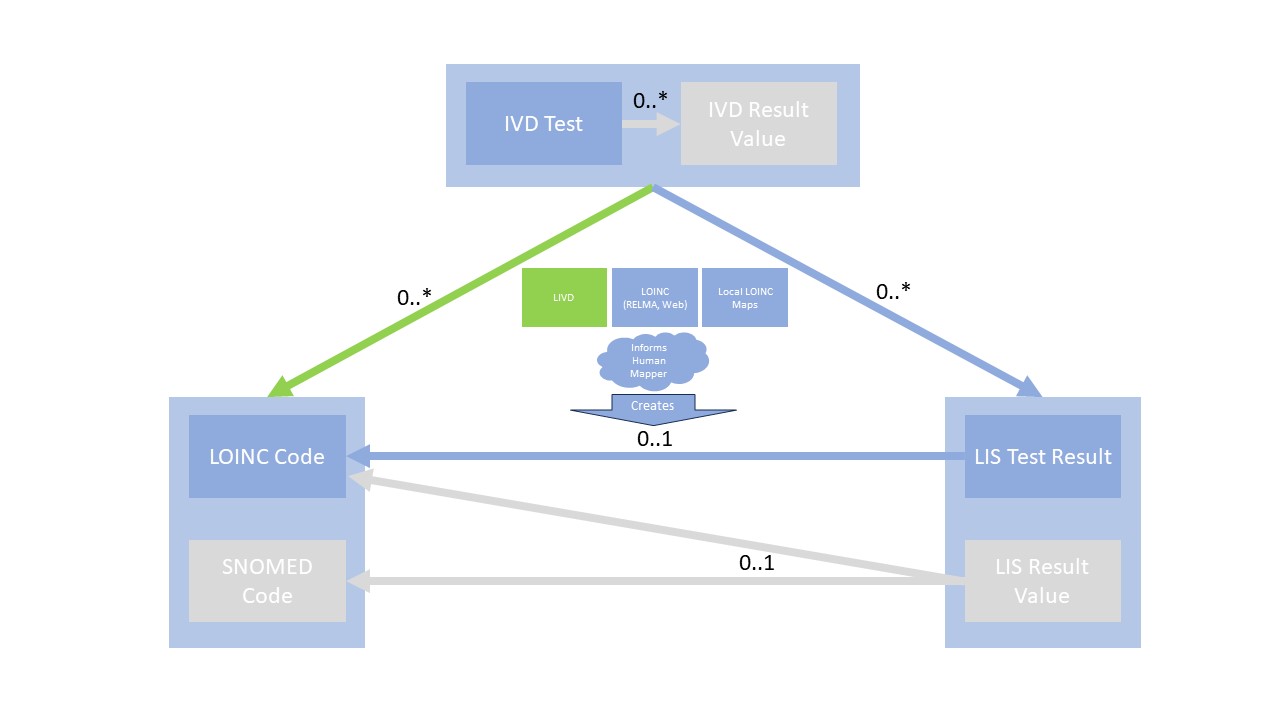
Note: The IVD Result Value to LOINC answer codes and SNOMED codes to support LIS Result Values to the codes are of of scope for this version, therefore greyed-out.
When the laboratory professional builds the test results that their LIS will manage and interact with the devices, they can use the device vendor’s suggestions to more accurately, consistently, and efficiently map the results in their LIS to a LOINC code in context of the device’s IVD Test. Note that, as these are device vendor’s suggestions, the expectation is that the suggested mappings are displayed and assist the laboratory staff to narrow the likely options that fit the laboratory’s intended use based. Therefore, the mappings are not suitable for automated configuration. The following example clarifies the information a laboratory professional would use during their configuration.
Vendor Analyte Code = 1067 (Gluc) is mapped in the LIS to:
LIVD Mapping Vendor Analyte Code = 1067 (Gluc) suggests:
Consequently, most appropriate mapping:
This IVD “test kit” is usually named Total Protein (CSF/Urine) and utilized for the analysis of CSF, Random/Spot Urine, or Timed urine (24 hr, 2 hr, etc) specimens. It is often used to perform body fluid Total Proteins. However, a different IVD “test kit” is typically used for serum/plasma Total Protein levels, which is out of scope for this example. Note that the LIVD file would NOT contain the local Ask at ORder entry questions (local codes 444 and 555), nor the calculated value for local code 777. CSF=Cerebrospinal Fluid. Vendor Analyte Code = 1099 (Total Protein CSF/Ur) is mapped in the LIS to:
LIVD Mapping Vendor Analyte Code = 1099 (Total Protein CSF/Ur) suggests:
Consequently, most appropriate mapping would be:
A manufacturer defined the following codes for an Immunoassay molecular antigen test:
The manufacture established the following SNOMED CT mappings for the result values: