This page is part of the Respiratory Virus Hospitalization Surveillance Network (RESP-NET) Content Implementation Guide (v1.0.0-ballot: STU1 Ballot 1) based on FHIR (HL7® FHIR® Standard) R4. The current version which supersedes this version is 1.0.0. For a full list of available versions, see the Directory of published versions
Respiratory Virus Hospitalization Surveillance Network (RESP-NET) cases are currently identified through a variety of mechanisms at each site, including review of daily or weekly hospital and laboratory line lists and reportable disease databases, through an often manual and labor-intensive process. Additionally, for laboratory-confirmed hospitalized cases identified through RESP-NET, surveillance staff conduct medical chart abstractions based on a standardized case report form to obtain detailed demographic and clinical data on hospitalized cases including length of stay, underlying medical conditions, hospital course, and in-hospital deaths.
CDC seeks to expand the utilization of electronic health information in RESP-NET and improve data exchange between healthcare facilities, state/local public health partners, and CDC to maintain the quality of these important surveillance activities while improving the efficiency and timeliness with which these data are available. Such enhancements will reduce the burden on surveillance sites and allow for scalability to additional sites in the future.
The goals of the RESP-NET reporting use cases include:
The following actors from the MedMorph RA IG are used by the RESP-NET use cases:
This section outlines the high-level interactions between the various MedMorph Actors and Systems listed above. There are two ways for a RESP-NET report to be created. The first is to use the MedMorph HDEA which is described in Figure 2.1 below. Figure 2.2 is for a Data Source who wishes to use their own functionality (not the HDEA) to create a RESP-NET report.
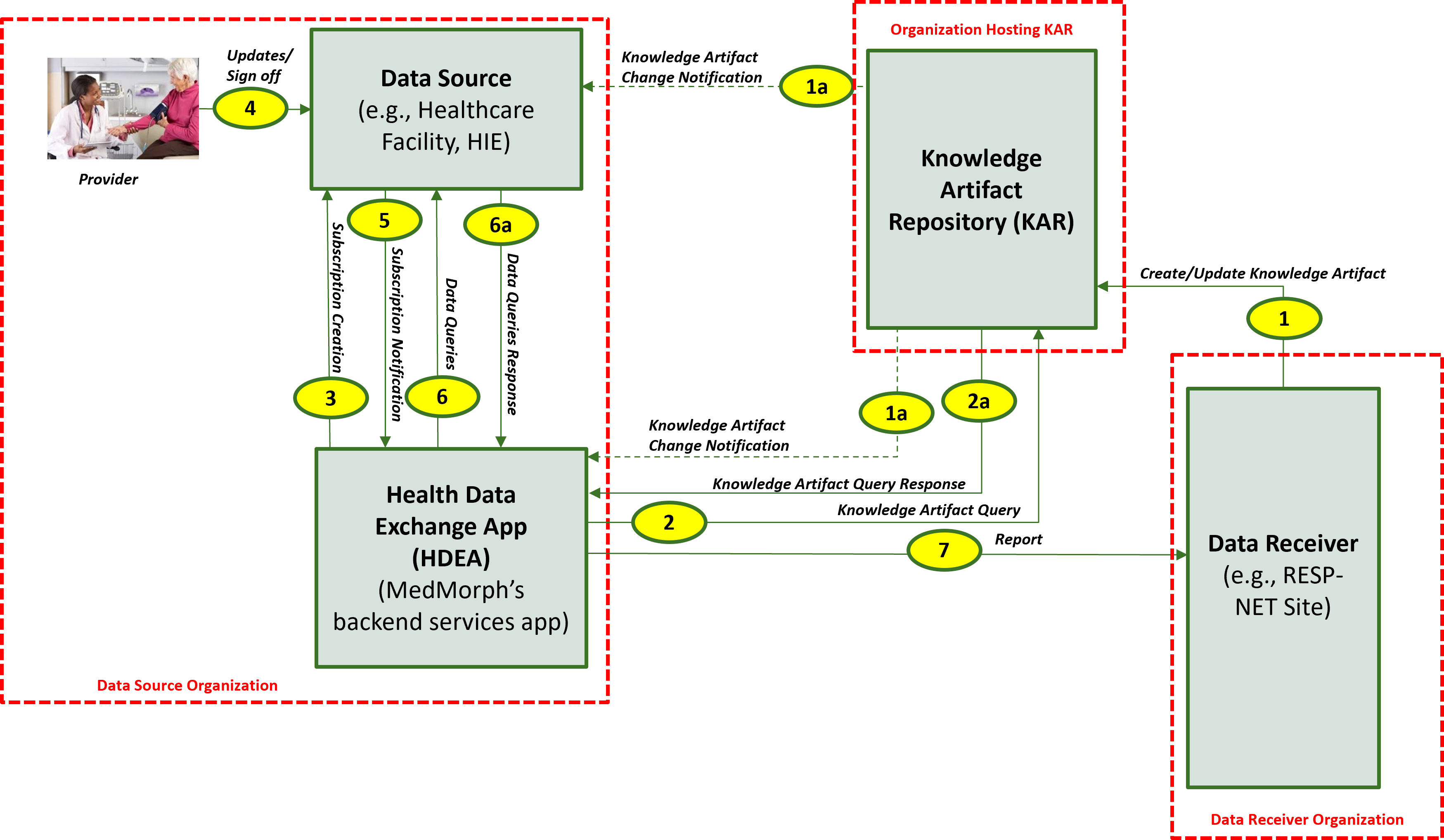
The descriptions for each step in the above diagram include:

For a Data Source that doesn’t want to use the HDEA, we have provided an alternate workflow for creating a RESP-NET report. The descriptions for each step in the above diagram include:
The descriptions for each step in the above diagram include:
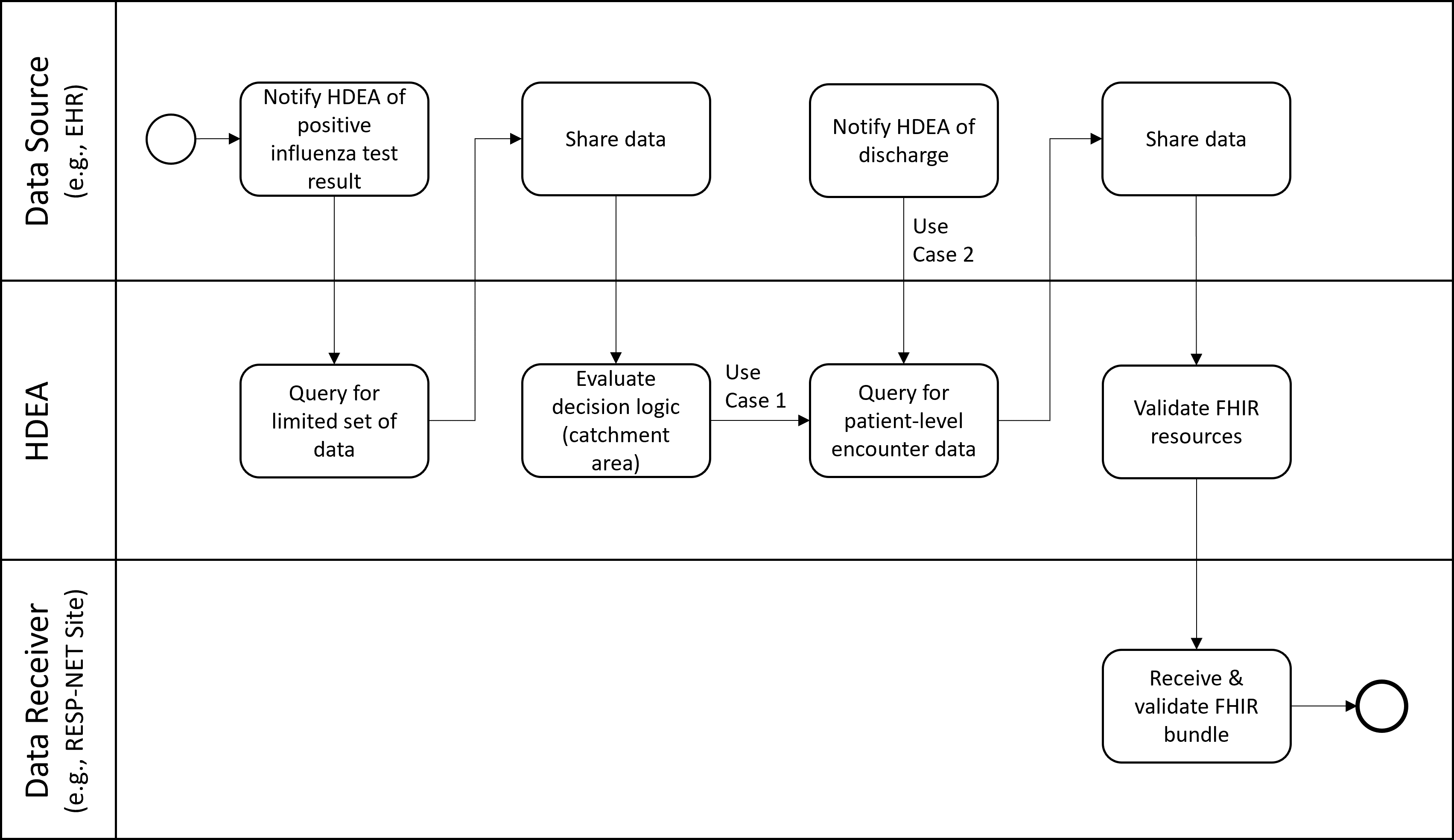
These use cases will identify patients hospitalized with laboratory-confirmed influenza among residents of the FluSurv-NET catchment area (defined by patient county of residence) for active population-based surveillance. The cases are reported along with minimum patient-level data and transmitted to the FluSurv-NET surveillance officers in the relevant state/local jurisdiction.
Use Case 1 is reported within one week of case identification. Minimum data elements include state and county of residence (or other geographic regions) to define the catchment area, patient age or date of birth, sex, race/ethnicity, hospital admission date, positive influenza testing data (test type, test result, test date), and influenza type and subtype (if available).
Use Case 2 focuses on the more detailed clinical surveillance data collected in FluSurv-NET by site surveillance officers. Patient-level data from EHRs about persons hospitalized with influenza include data on important outcomes and indicators of disease severity such as ICU admission, mechanical ventilation, in-hospital death, and length of hospital stay. Additional elements include health history (underlying conditions, tobacco, alcohol, substance use), clinical course, viral and bacterial codetections, findings from chest imaging, diagnoses, vaccination, and treatment. This use case will include person-level clinical data elements that are currently collected in FluSurv-NET on a standardized case report form for all identified cases. Not all of the current data elements may be reachable in a FHIR-based approach; proposed solutions should recognize that this may evolve over time and surveillance officers may still need to conduct medical chart review on data elements.
The positive influenza test results in a notification to the HDEA/BSA. The HDEA queries for a limited set of PII, including the patient’s address. If the patient is not a resident of a catchment area, the HDEA stops all activity regarding this patient. If the patient is a resident of a catchment area the HDEA then regularly monitors the patient’s record until discharge occurs. Upon discharge, the HDEA requests a set of FHIR resources representing patient-level encounter information from the Data Source. This limited data set includes PII and other data. The obtained resources are validated (e.g., checked to see if they are conformant to the appropriate FHIR profiles), bundled, and transmitted to the RESP-NET Site.
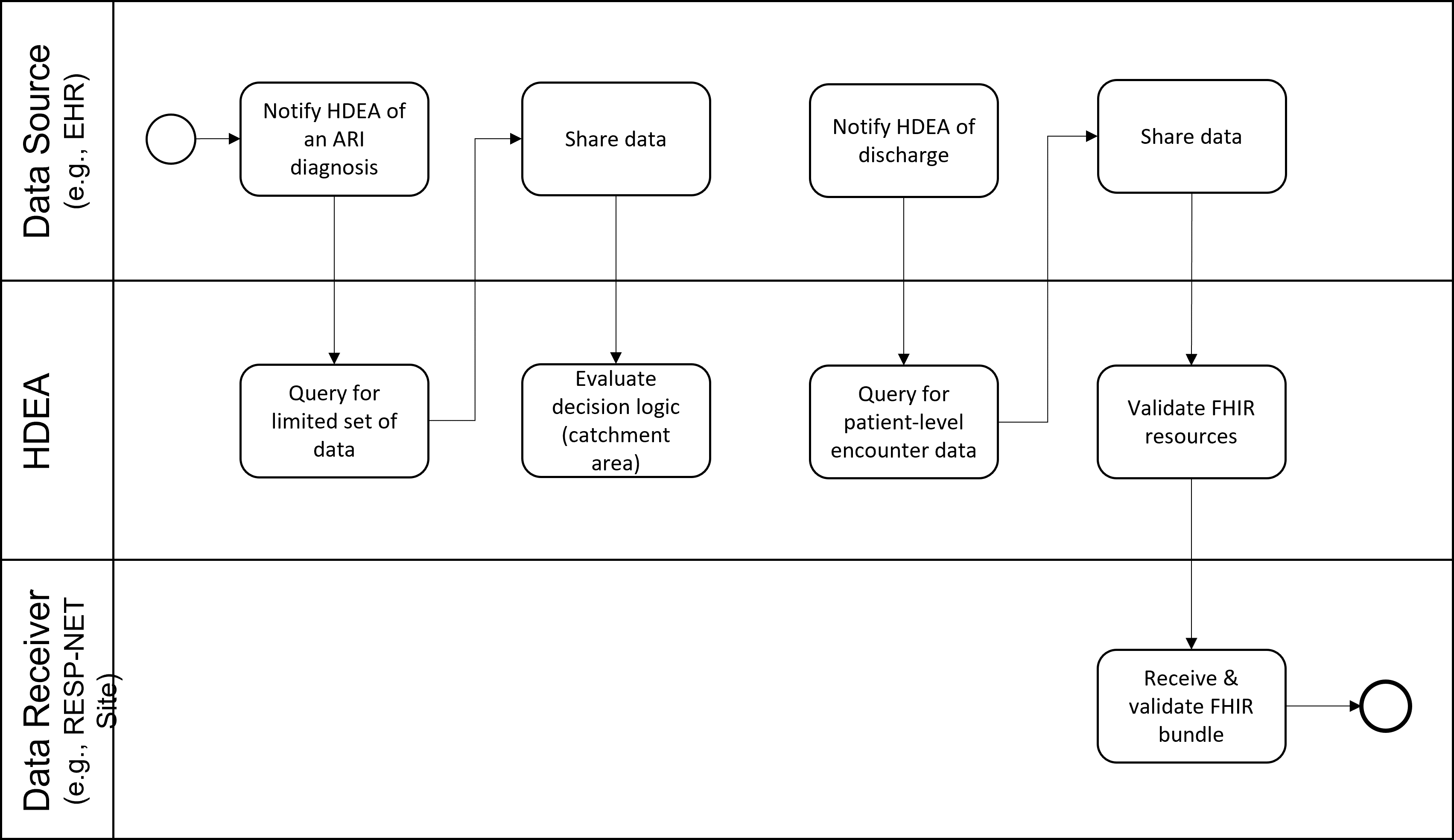
Because testing for influenza and other respiratory viruses is done at the discretion of the clinician and testing practices may vary widely among facilities and over time, some people hospitalized with influenza may not be recognized and diagnosed. To better estimate the full burden of influenza, CDC collects additional information from FluSurv-NET hospitals on the proportion of patients with acute respiratory illnesses (ARI) who are tested for influenza, SARS-CoV-2, and RSV. This use case will focus on obtaining data on respiratory viral testing practices for CDC’s influenza disease burden project and transmitting it directly to CDC throughout the season. This project collects anonymized patient-level data on persons hospitalized with ARI (based on ICD-10 diagnosis codes) along with information about whether or not the patient was tested for influenza, SARS-CoV-2, or RSV and if so, the test result and test type used for all tests performed (patients may be tested multiple times). Additional data elements include state, age, race/ethnicity, week of admission, ICD-10 discharge diagnoses, and if possible, whether the patient had been admitted to the ICU or died during the hospital stay.
The diagnosis results in a notification to the HDEA/BSA. The HDEA queries for a limited set of PII, including the patient’s address. The HDEA then regularly monitors the patient’s record until discharge occurs. Upon discharge, the HDEA requests a set of FHIR resources representing patient-level encounter information from the Data Source. This limited data set includes PII and other data. The data reported includes testing status for influenza, SARS-CoV-2, and RSV occurred. The obtained resources are validated (e.g., checked to see if they are conformant to the appropriate FHIR profiles), bundled, and transmitted to the RESP-NET site.
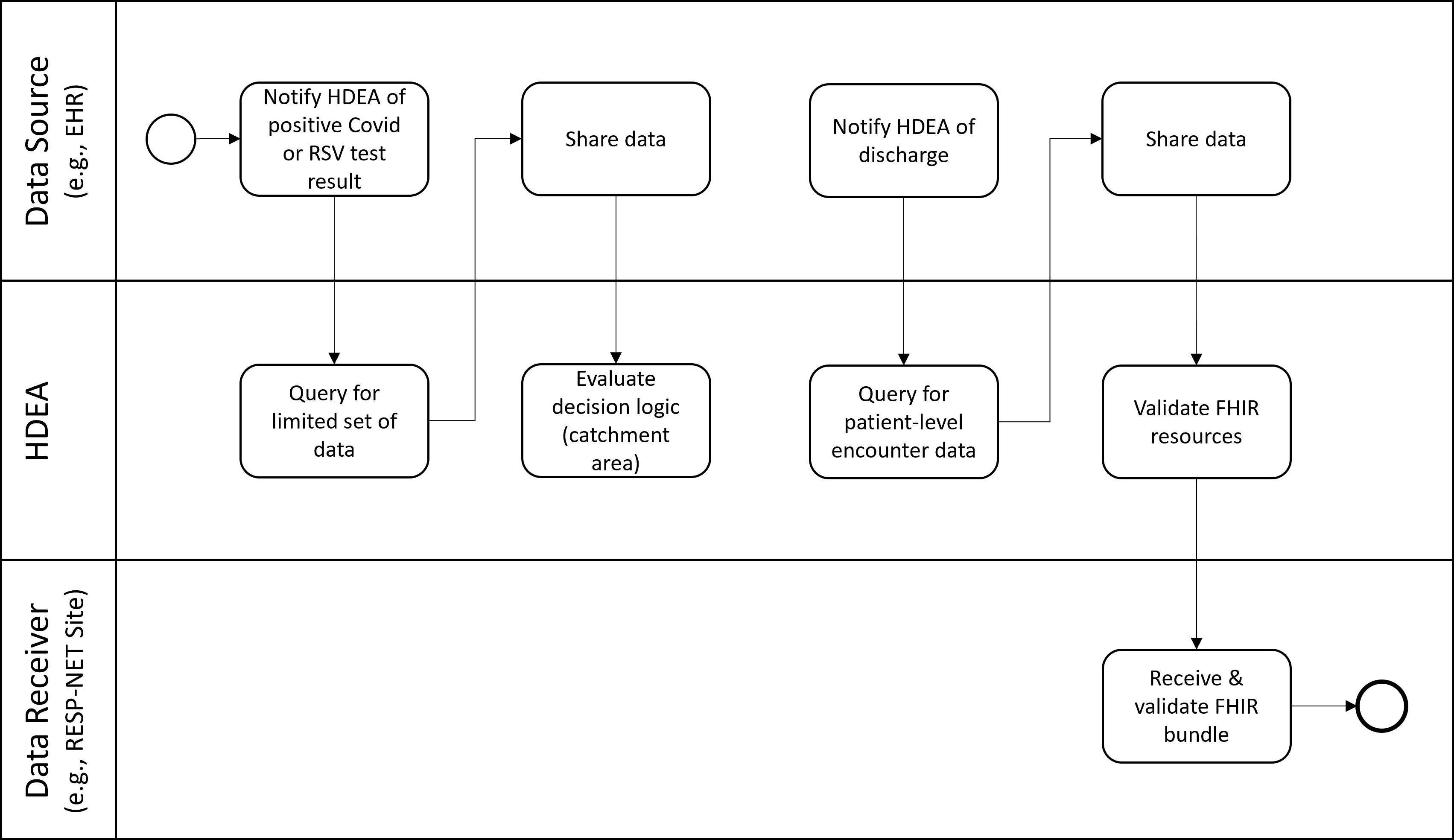
These use cases focus on collecting equivalent data to Use Cases 1-2 but for patients who test positive for SARSCoV-2 (the virus that causes COVID-19) or Respiratory Syncytial Virus (RSV). Public health surveillance through COVID-NET and RSV-NET is conducted in the same sites as FluSurv-NET, collects many of the same data elements, with some additional data elements specific to these viral illnesses.
The positive COVID or RSV test results in a notification to the HDEA/BSA. The HDEA queries for a limited set of PII, including the patient’s address. If the patient is not a resident of a catchment area, the HDEA stops all activity regarding this patient. If the patient is a resident of a catchment area the HDEA then regularly monitors the patient’s record until discharge occurs. Upon discharge, the HDEA requests a set of FHIR resources representing patient-level encounter information from the Data Source. This limited data set includes PII and other data. The obtained resources are validated (e.g., checked to see if they are conformant to the appropriate FHIR profiles), bundled, and transmitted to the RESP-NET Site.