This page is part of the PACIO Advance Directive Information Implementation Guide (v0.1.0: STU 1 Ballot 1) based on FHIR (HL7® FHIR® Standard) R4. The current version which supersedes this version is 1.0.0. For a full list of available versions, see the Directory of published versions
This section defines additional requirements and guidance relevant to this guide as a whole. The conformance verbs - SHALL, SHOULD, MAY - used in this guide are defined in FHIR Conformance Rules.
To claim conformance to a profile in this guide, servers SHALL:
The following rules apply to all PACIO ADI Profile elements marked as Must Support. Must Support on any profile data element SHALL be interpreted as follows:
Profiles used by this guide, but defined in other implementation guides inherit the definition of Must Support from their respective guides.
The area of advance directive interoperability is relatively new and codes capturing the concepts related to advance directives are not well established or well known. This implementation guide provides several codes for expressing this information but specifies extensible bindings to use other code systems where necessary. These code systems may also not be well-known. Additionally, there are not widely accepted universal or national for standards for capturing this information. Different scopes of use and jurisdictions capture and organize this information in different ways. As such, it is important for data sources to capture this information as it is presented and for data consumer systems to be able to present it the same way to users.
To that end, several CodeableConcept text data elements are marked as Must Support.
Per the FHIR Standard for Using Text in CodeableConcept:
The
textis the representation of the concept as entered or chosen by the user, and which most closely represents the intended meaning of the user or concept. Very often thetextis the same as adisplayof one of the codings. One or more of the codings may be flagged as the user selected code - the code or concept that the user selected directly.
In some cases a code may not exist for a particular concept, in such cases, it is possible to provide a free text only representation of the concept in the CodeableConcept text element without any ‘coding’ elements present.
For example, using text only, the Goal.category element would be:
"valueCodeableConcept": {
"text": "Free text concept description"
}
Because advance directive interoperability is relatively new and there are not any widely accepted universal or national for standards for capturing this information, advance directives may be represented in many different ways. It is important that this information be communicated as it is meant and that it is received and viewable in that same manner.
To address this need, most of the profiles in this implementation guide require the resource instance’s text element (cardinality 1..1).
The text element of a resource is a Narrative data type. The status element of this data type indicates whether the text is generated by a system based on the structured data in the resource or if it contains additional information. The status element is required.
For the purposes of this implementation guide, it is expected that most implementations will have resource instances that have additional data in the text than is captured in the structured data. When that is the case, the narrative text.status SHALL be additional.
This guide requires the interoperability of Advance Directive Information through the use of wholly contained documents as part of its use case. While it is required that this data be made interoperable as a collection of Advance Directive Information in document Bundles, systems may decide to make use of the constituent resources as separate resources for additional uses and purposes, such as use in support of Clinical Decision Support.
Advance directive documents may take several forms including scanned PDF documents, CDA documents and native FHIR documents. This guide defines interoperability to support any number of types, though focuses on native FHIR documents.
All document content, regardless of format is saved in the Binary resource and is available through the Binary endpoint. FHIR native documents SHALL be Bundle resources with type = document and encoded as a Binary resource. Documents that are communicated SHALL have at least one DocumentReference resource that references the Binary though the DocumentReference.content.attachment.url.
FHIR native documents SHOULD have all content contained within the Bundle with no external references except for the references to external documents in the DocumentationObservation.focus. FHIR native documents have internal references between resources (e.g. the Composition resource referencing entry resources). These references SHALL be resolved using the Bundle.entry.fullUrl. This URL may be a proper URL, but there SHOULD be no expectation that the URL resolves outside of the confines of the Bundle. To avoid confusion, it may be desireable to use UUID (e.g. urn:uuid:53fefa32-fcbb-4ff8-8a92-55ee120877b7) instead of URLs for the fullUrl.
Digital signatures provide trusted signatures, non-repudiation, and they make the signed document tamper-proof. Digital signatures include several capabilities that distinguish them from basic electronic signatures. In much the same way as other document exchange, the use of digital signatures for advance directive document interoperability will depend on the specific scenario, parties involved, and jurisdictional or organizational requirements. There are challenges and costs to using digital signatures that may present an unnecessary barrier to some applications of this implementation guide. Therefore this guide does not require the use of digital signatures, but instead recommend that systems SHOULD support digital signatures where possible.
There are a number of options available for signing documents in FHIR. For context, below is a description of a number that were considered for this guide.
The first option is an embedded signature where a FHIR document bundle is digitally signed. In this method, the digital signature is saved in Bundle.signature. The result is the content and document bundle are included in a single DocumentReference Resource. This approach works for JSON and xml bundles.
The second option is a detached signature, in which the Binary Resource is digitally signed and saved as a new DocumentReference. This option results in two DocumentReferences where one DocumentReference has the content and the other contains the digital signature. The DocumentReference with the signature uses DocumentReference.relatesTo.code were the code is signs and DocumentReference.relatesTo.target points to the DocumentReference that contains the content. The detached signature option supports FHIR Document Bundle, CDA, pdf, and mp3 content.
The final option uses an enveloped signature. In this option, the content is wrapped in a FHIR Bundle in a JSON object and then encoded to binary. The binary is then signed using JSON Web Signature. The enveloped signature described only allows for signing of JSON files.
If digital signatures are supported, the method of signatures this guide specifies is the detached signature. The detached signature approach provides the greatest flexibility of document type support (does not have to be a JSON or even a FHIR encoded document) and enables the ability for clients that do not support or require digital signatures to retrieve and use the data unhampered. The detached signature approach is also aligned with the design and workflows of major US health information networks. Systems claiming conformance to this guide that support digital signatures SHALL support the detached signature stored in a separate Binary resource and referenced by a DocumentReference resource as described below.
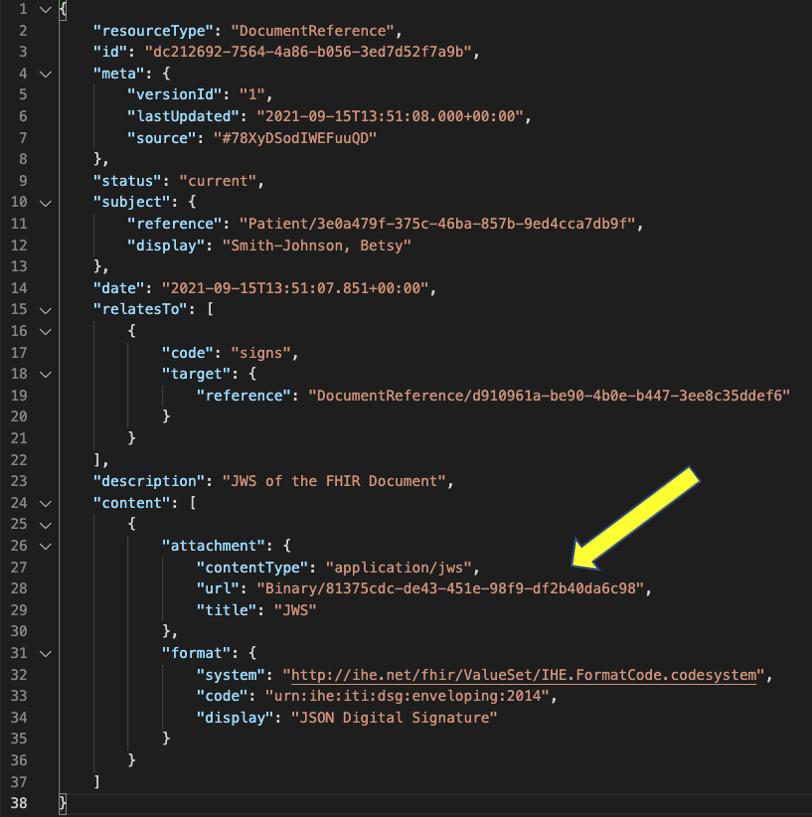
For the detached signature, the digital signature is saved in a Binary resource and pointed to from an additional DocumentReference resource in the DocumentReference.content.attachment.url. This signature DocumentReference links to the advance directive document DocumentReference using DocumentReference.relatesTo.target and the code signs is used in the DocumentReference.relatesTo.code field to identify how this DocumentReference resource relates to the target.
The cryptographic digital signature is included in the DocumentReference.content as an attachment. The mimeType for the digital signature is Content.attachment.contentType.application/jws for json signature and Content.attachment.contentType.xml-signature” for xml signature. The details of the cryptographic digital signature SHALL be a referenced Binary Resource using url.
Below is an example of detached digital signature with the cryptographic digital signature referenced using Binary Resource.
At some point it may be necessary for an advance directive document to be replaced or deprecated. The situations and conditions in which a document is replaced or deprecated is dependent on jurisdictional requirements as well as the intent and interests of the actors involved. When a document is replaced or deprecated is beyond the scope of this guide, however, this guide does specify requirements that need to be supported in the event documents need to be replaced or deprecated.
When a document is to be replaced, a new DocumentReference is created. The new document points “backwards” to the documentReference it is replacing through the DocumentReference.relatesTo.target and a DocumentReference.relatesTo.code with a value of replaces. The original document then should be marked as replaced by updating the DocumentReference.status to the code superseded. Ideally the DocumentReference being replaced and the replacing DocumentReference will have matching business identifiers (DocumentReference.identifier) to enable direct searching across multiple versions.
Documents can be deprecated through a document replacement that includes a new version indicating that the document is deprecated or otherwise no longer in force.