This page is part of the Da Vinci Data Exchange for Quality Measures (DEQM) FHIR IG (v1.0.0: STU 1) based on FHIR (HL7® FHIR® Standard) R3. The current version which supersedes this version is 5.0.0. For a full list of available versions, see the Directory of published versions
StructureDefinition-deviceusestatement-deqm
The DEQM DeviceUseStatement Profile describes a shareable, published device use statement that can be used to define an application of a device
Mandatory Data Elements and Terminology
The following data-elements are mandatory (i.e data MUST be present).
Each DeviceUseStatement must have:
- The [Not Done] extension
- Note that this guide extends the context of use of this extension to the DeviceUseStatement.
Formal Views of Profile Content
Description of Profiles, Differentials, and Snapshots.
The official URL for this profile is:
http://hl7.org/fhir/us/davinci-deqm/StructureDefinition/deviceusestatement-deqm
Published on Tue Jun 19 00:00:00 UTC 2018 as active by Da Vinci Project.
This profile builds on QICore-DeviceUseStatement
| Name | Flags | Card. | Type | Description & Constraints |
|---|---|---|---|---|
 | I | 0..* | QICore-DeviceUseStatement | Record of use of a device |
  | Σ | 0..1 | id | Logical id of this artifact |
  | Σ | 0..1 | Meta | Metadata about the resource |
  | ?!Σ | 0..1 | uri | A set of rules under which this content was created |
  | 0..1 | code | Language of the resource content Binding: Common Languages (extensible) Max Binding: All Languages | |
  | I | 0..1 | Narrative | Text summary of the resource, for human interpretation |
  | 0..* | Resource | Contained, inline Resources | |
  | 0..* | Extension | Extension Slice: Unordered, Open by value:url | |
  | ?!SI | 0..1 | boolean | Event did not occur URL: http://hl7.org/fhir/StructureDefinition/event-notDone |
  | ?! | 0..* | Extension | Extensions that cannot be ignored |
  | 0..* | Identifier | External identifier for this record | |
  | ?!SΣ | 1..1 | code | active | completed | entered-in-error + Binding: DeviceUseStatementStatus (required) |
  | S | 1..1 | Reference(QICore-Patient) | Patient using device |
  | S | 0..1 | Period | Period device was used |
  | S | 0..1 | How often the device was used | |
 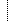  | Timing | |||
 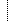  | Period | |||
 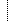  | dateTime | |||
  | S | 0..1 | dateTime | When statement was recorded |
  | 0..1 | Reference(Patient | Practitioner | RelatedPerson) | Who made the statement | |
  | S | 1..1 | Reference(QICore-Device) | Reference to device used |
  | S | 0..* | CodeableConcept | Why device was used Binding: SNOMED CT Clinical Findings (preferred) |
  | S | 0..1 | CodeableConcept | Target body site Binding: SNOMED CT Body Structures (example) |
  | 0..* | Annotation | Addition details (comments, instructions) | |
 Documentation for this format Documentation for this format | ||||
Differential View
Snapshot View
| Name | Flags | Card. | Type | Description & Constraints |
|---|---|---|---|---|
 | I | 0..* | QICore-DeviceUseStatement | Record of use of a device |
  | Σ | 0..1 | id | Logical id of this artifact |
  | Σ | 0..1 | Meta | Metadata about the resource |
  | ?!Σ | 0..1 | uri | A set of rules under which this content was created |
  | 0..1 | code | Language of the resource content Binding: Common Languages (extensible) Max Binding: All Languages | |
  | I | 0..1 | Narrative | Text summary of the resource, for human interpretation |
  | 0..* | Resource | Contained, inline Resources | |
  | 0..* | Extension | Extension Slice: Unordered, Open by value:url | |
  | ?!SI | 0..1 | boolean | Event did not occur URL: http://hl7.org/fhir/StructureDefinition/event-notDone |
  | ?! | 0..* | Extension | Extensions that cannot be ignored |
  | 0..* | Identifier | External identifier for this record | |
  | ?!SΣ | 1..1 | code | active | completed | entered-in-error + Binding: DeviceUseStatementStatus (required) |
  | S | 1..1 | Reference(QICore-Patient) | Patient using device |
  | S | 0..1 | Period | Period device was used |
  | S | 0..1 | How often the device was used | |
   | Timing | |||
   | Period | |||
   | dateTime | |||
  | S | 0..1 | dateTime | When statement was recorded |
  | 0..1 | Reference(Patient | Practitioner | RelatedPerson) | Who made the statement | |
  | S | 1..1 | Reference(QICore-Device) | Reference to device used |
  | S | 0..* | CodeableConcept | Why device was used Binding: SNOMED CT Clinical Findings (preferred) |
  | S | 0..1 | CodeableConcept | Target body site Binding: SNOMED CT Body Structures (example) |
  | 0..* | Annotation | Addition details (comments, instructions) | |
 Documentation for this format Documentation for this format | ||||
Downloads: StructureDefinition: (XML, JSON), Schema: XML Schematron
Quick Start
Below is an overview of the required set of RESTful FHIR interactions - for example, search and read operations - for this profile. See the Conformance requirements for a complete list of supported RESTful interactions for this IG.
Use cases:
- currently none defined



