This page is part of the FHIR for FAIR - FHIR Implementation Guide (v1.0.0: STU 1) based on FHIR v4.3.0. This is the current published version in its permanent home (it will always be available at this URL). For a full list of available versions, see the Directory of published versions 
This guide has been designed following an incremental, iterative and meet-in-the -middle approach.
In this sense authors do not pretend to cover in this version of the guide all the aspects related to the data FAIRification (e.g., security and privacy) or all the possible kind of data.
The intent of this guide is to promote as possible the reuse of existing artefacts, in this sense no new FHIR profiles have been specified when covered by existing guides (e.g. genomic, lab results, vital sign).
This guide is not about FAIR in general, but on how HL7 FHIR should be used to better support the FAIR principles. The design choices will be therefore based on how FHIR is designed: this may imply that some FAIR expectations might not be fully accomplished or realized under specific conditions.
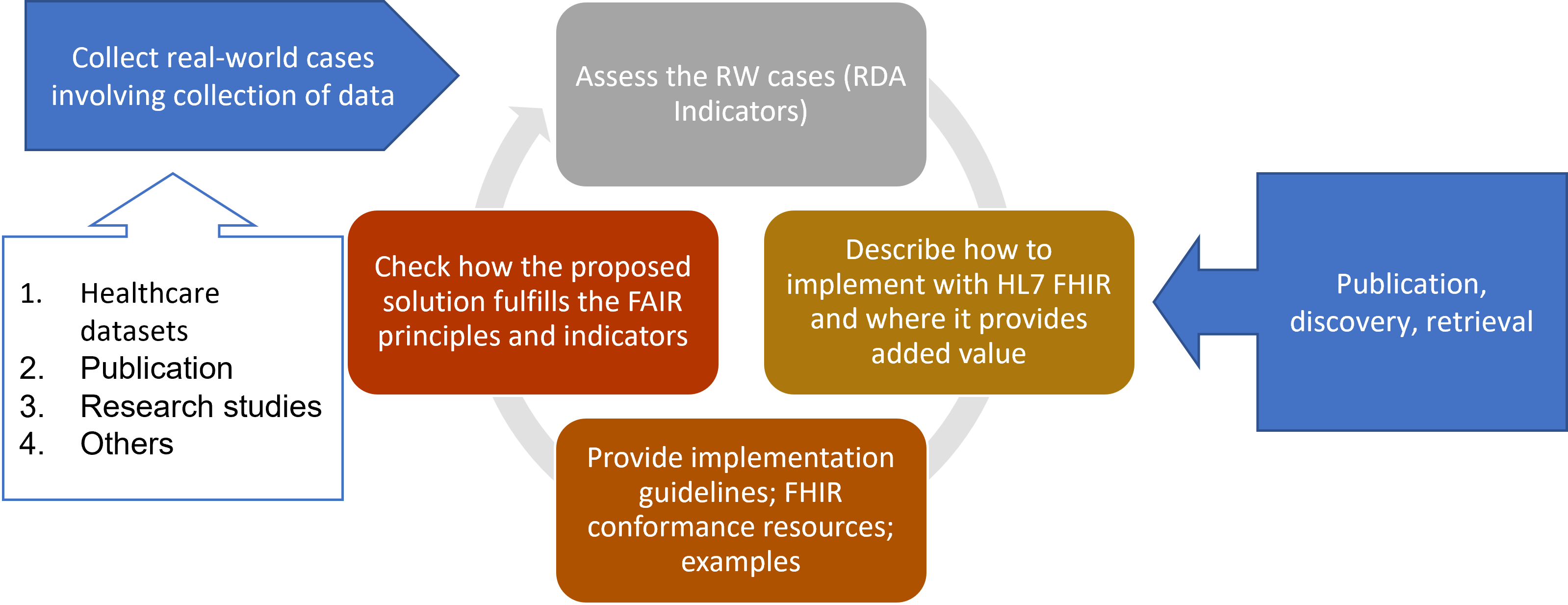
The following figure shows how this incremental, iterative and meet-in-the -middle approach has been realized. A set of representative real-world study cases have selected covering different situations: research studies, publications, sharing of health data and others. For each selected case (iteratively):
1) The case has been analyzed, performing a FAIRness assessment against the RDA indicators, and identifying the main findings and gaps; and/or capturing the main lesson learned by the implementation of the FAIR principles.
2) It has been analyzed how these cases are or could be implemented by using HL7 FHIR and where it provides added value.
3) A set of guidelines for implementing FAIR principles with HL7 FHIR has been produced and FHIR conformance resources to represent subject and study level metadata have been specified.
4) Finally, it has been checked how the proposed solution fulfills the FAIR principles and RDA indicators
 |