This page is part of the FHIR Clinical Documents (v1.0.0-ballot: STU1 Ballot 1) based on FHIR (HL7® FHIR® Standard) R4. The current version which supersedes this version is 1.0.0. For a full list of available versions, see the Directory of published versions
| Page standards status: Informative |
The FHIR Clinical Document IG was originally derived from the HL7 Clinical Document Architecture, and was developed in part to resolve CDA to FHIR mapping discrepancies found across a number of FHIR-based artifacts. Landscape analysis leading up to the development of this IG revealed HUNDREDS of existing Bundle and Composition profiles, many of which are designed for clinical documents, with no clear canonical pattern upon which future FHIR-based clinical documents work should be based. Existing profiles and IGs take many different approaches to representing various CDA constructs.
The FHIR Clinical Document IG is aligned with CDA, but not a mirror image of CDA. While this IG is based on CDA and will enable a straightforward mapping from CDA to FHIR, it will not necessarily provide a reverse mapping. Future IG updates will assess the need for additional profiles, and may add features that go beyond what is capable with today's CDA.
Scope for this version of the IG is limited to FHIR artifacts reflecting the CDA Header. More precisely, and as shown in the following figure within the red boxes, scope includes FHIR artifacts corresponding to:
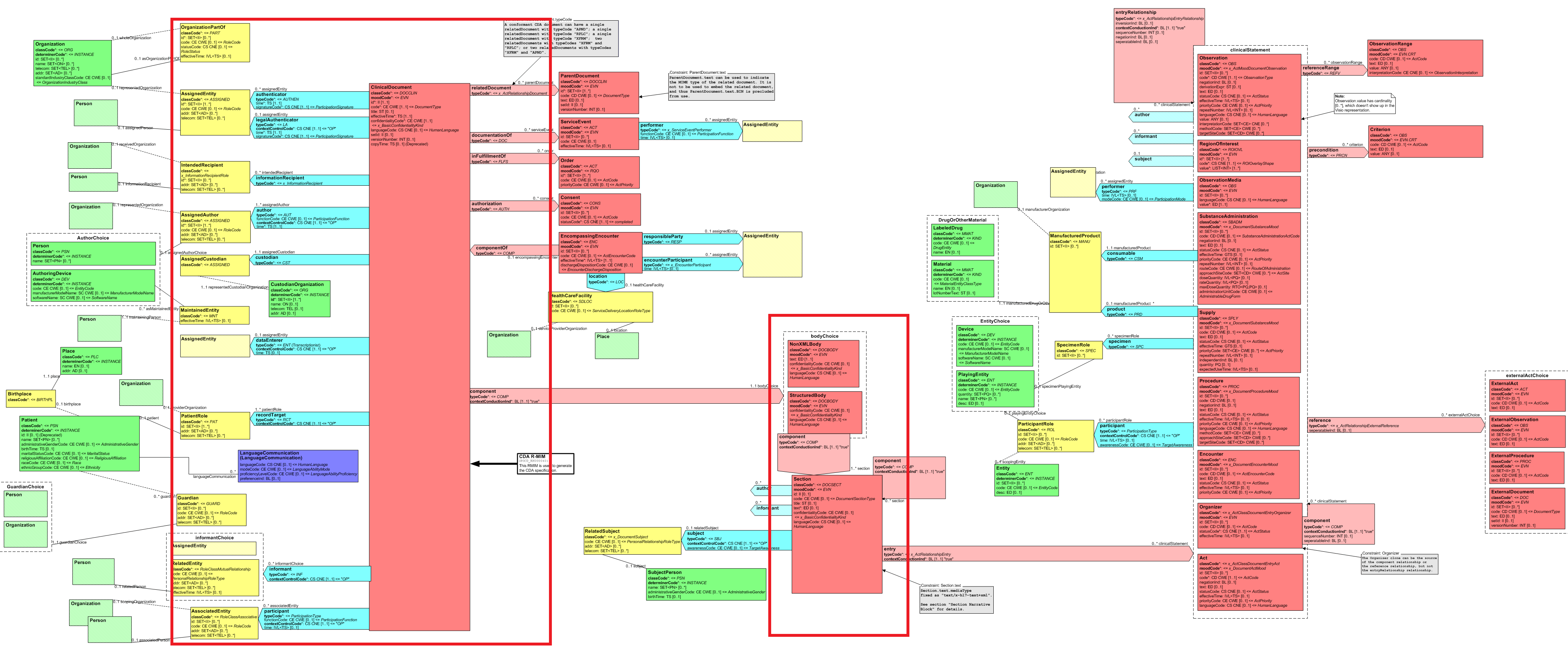
Detailed mappings of this IG can be found on the Profile Structure Definitions. See Composition and Bundle mappings.
A high level mapping from CDA to FHIR Clinical Document is shown here. Composition attributes in bold have a minimal cardinality of 1 in the core resource. Composition attributes in green represent extensions. CDA extensions that map into the FHIR bundle and composition (prefixed with "sdtc:") are also shown.
We are seeking feedback on the CDA NonXMLBody mapping. One can communicate an unstructured document such as a PDF as the target of a DocumentReference OR one can convert the unstructured document to a FHIR Clinical Document which then serves as the target of a DocumentReference. Current guidance allows either approach.