This page is part of the Electronic Medicinal Product Information (ePI) FHIR Implementation Guide (v1.0.0: STU1) based on FHIR v5.0.0. This is the current published version. For a full list of available versions, see the Directory of published versions 
The following is an example of how to build a Summary of Product Characteristics (SmPC) as a Type 1, Type 2, and Type 3 ePI document. This example is meant as a demonstration to encourage a harmonized approach. Refer to national or regional health authority guidance for official rules about how to build an ePI document.
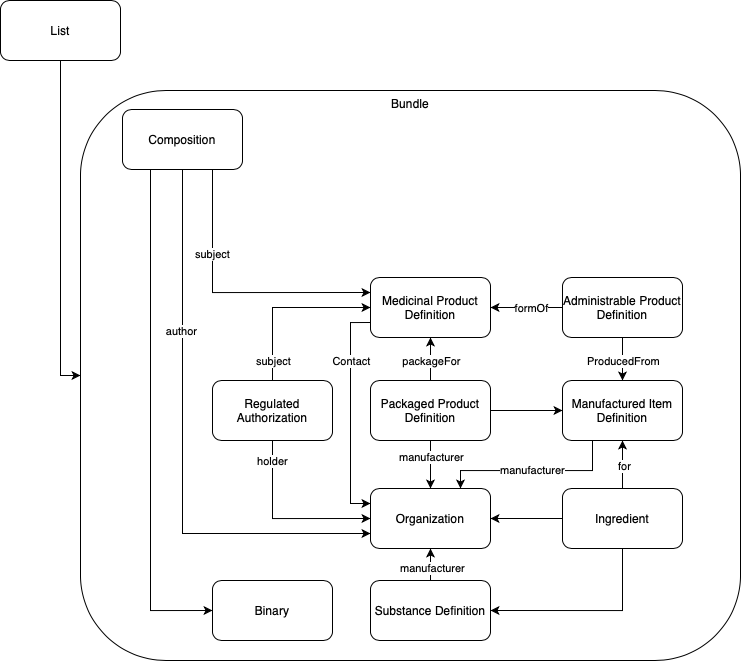
Using the Base ePI Profile as a template, complete one Organization resource for each organization associated with the authorized product(s) in this ePI. The following are examples of the type of organization typically associated with ePIs: Market Authorization Holder; health authority responsible for regulating the ePI content; manufacture, test, analysis, packaging).
Refer to the Organization profile for detail.
Using the Base ePI Profile as a template, complete one SubstanceDefinition resource for each active ingredient associated with the authorized product(s) in this ePI.
Create a reference from the SubstanceDefinition to the Organization resource for the manufacturer or marketing authorization holder that was created in the Step 5.1.1 Create Organization resource(s)].
Refer to the SubstanceDefinition Profile for detail.
The manufactured item describes the medicinal product as the dosage form contained in its primary package. For example, a powder in a vial and a diluent in another vial are packaged together in a kit. The powder is one manufactured item and the diluent is a second manufactured item.
Using the FHIR ePI Profile as a template, complete one ManufacturedItemDefinition resource for each manufactured item associated with the authorized product(s) in this ePI.
Create a reference from each ManufacturedItemDefinition resource to the corresponding Organization resource (e.g., reference to the manufacturer; reference to the marketing authorization holder).
Refer to ManufacturedItemDefinition Profile for detail.
(add two examples, one for a product with no transformation (tablet) and one with transformation).
Using the Base ePI Profile as a template, complete one Ingredient resource for each active ingredient and each excipient that make up each manufactured item associated with the authorized product(s) in this ePI.
Create a reference from the relevant Ingredient resources to the corresponding ManufacturedItemDefinition resource (e.g., reference the ingredients that make up the powder to the powder’s manufactured item, and reference the ingredients that make up the diluent to the diluent’s manufactured item).
Create a reference from the relevant Ingredient resources to the corresponding Organization resource (e.g., reference to the Organization that manufactures that ingredient).
Create a reference from the relevant Ingredient resources to the corresponding Substance Definition resource (e.g., reference the active ingredient to the corresponding substance so there is a link to capture molecular structure, molecular formula, and molecular weight.).
Refer to Ingredient for detail.
Using the FHIR ePI Profile as a template, complete one MedicinalProductDefinition resource for each presentation of the medicinal product(s) associated with the authorized product(s) in this ePI.
There are no references from the medicinal product to other resources. Instead, other resources will refer to the medicinal product.
Refer to MedicinalProductDefinition Profile for detail.
The administrable product describes the medicinal product in the dosage form ready for administration to the patient (after any mixing of multiple components or transformations has been performed). This is different from the manufactured item which described the medicinal product as the dosage form in the primary packaging and before any mixing or transformation. For example, a powder in a vial and a diluent in a vial are packaged together. The combined solution, made by mixing the powder and diluent, is the administrable product since that is the dosage form ready for administration to the patient.
Using the Base ePI Profile as a template, complete one PharmaceuticalProductDefinition resource for each pharmaceutical product associated with the authorized product(s) in this ePI.
Create a reference from each PharmaceuticalProductDefinition resource to the corresponding MedicinalProductDefinition resource.
Create a reference from each PharmaceuticalProductDefinition resource to the corresponding ManufacturedItemDefinition resource.
Refer to AdministrableProductDefinition Profile for detail.
(add two examples, one for a product with no transformation (tablet) and one with transformation).
Using the Base ePI Profile as a template, complete one PackagedProductDefinition resource for each presentation associated with the authorized product(s) in this ePI.
Create a reference from each PackagedProductDefinition resource to the corresponding MedicinalProductDefinition resource for this package.
Create a reference from each PackagedProductDefinition resource to the corresponding Organization resource for the manufacturer or marketing authorization holder.
Refer to PackagedProductDefinition Profile for detail.
Using the Base ePI Profile as a template, complete one RegulatedAuthorization resource for each medicinal product associated with this ePI. For example, if there are four medicinal products then there will be four RegulatedAuthorization resources.
Create a reference from each RegulatedAuthorization resource to its corresponding MedicinalProductDefinition.
Create a reference from each RegulatedAuthorization resource to to the corresponding Organization resource for the marketing authorization holder and the health authority.
Refer to RegulatedAuthorization Profile for detail.
NOTE:
Convert each image used in the ePI to Base64 (e.g., images used as figures; the chemical structure of the active ingredient).
Using the FHIR ePI Profile as a template, complete one Binary resource for each image in the ePI. Add the Base64 version of the image to the Binary resource.
The Binary can also be used to create a cross-reference linking to an outside object like a video.
Refer to Binary Resource for detail.
The composition captures all section headings, sub-section headings and narrative text/narrative content (e.g., paragraphs, tables, bulleted lists) in the ePI.
Using the FHIR ePI Profile as a template, complete one Composition resource for each ePI document.
Reference the Composition resource to each Binary. The reference to the Binary is made from the narrative text of the Composition resource’s @text element.
Reference the Composition resource to each Regulated Authorization from the @subject element.
The section/@Title is the display text of the section heading and sub-section heading prescribed by the relevant national health authority. For example, ‘2. Qualitative and quantitative composition’ or ‘4.1 Therapeutic indications’ from the EMA’s Quality Review of Document (QRD) template for the SmPC.
The section/@code is the code for the corresponding section heading or sub-section heading prescribed by the relevant national health authority.
NOTE:
ePI documents are meant to be separate and shall not be combined into one large composition. E.g.,
Refer to Composition Profile for detail.
The bundle is used to gather together the resources needed to create a unique ePI document. E.g., one bundle for the health care practitioner ePI; a second bundle for the patient insert ePI; a third bundle for the pack label ePI document.
Using the Base ePI Profile as a template, complete one Bundle resource for each ePI document.
Complete the Bundle resource by referencing it to only one Composition plus all other resources completed in Step 1. E.g., reference the Bundle to the patient insert composition and all other resources associated with that patient insert (e.g., the binaries, regulated authorizations, clinical uses, medicinal products, packaged products, administrable products, manufactured items, organizations, ingredients, substances).
NOTE:
Refer to Bundle Profile for detail.
The List of ePIs is used to keep track of all ePIs for a given medicinal product. E.g., the list will track the SmPC and all its versions; the Package Leaflet and all its versions.
Complete the List resource by adding a reference to the ePI document Bundle for the ePI document created above.