This page is part of the FHIR Specification (v4.2.0: R5 Preview #1). The current version which supercedes this version is 5.0.0. For a full list of available versions, see the Directory of published versions  . Page versions: R5 R4B R4
. Page versions: R5 R4B R4
Orders and Observations  Work Group Work Group | Maturity Level: 0 | Trial Use | Security Category: Anonymous | Compartments: Not linked to any defined compartments |
A kind of specimen with associated set of requirements.
SpecimenDefinition will define a kind of specimen, which can be associated with in vitro diagnostic procedures described in a catalog of orderable services. SpecimenDefinition describes the kind of specimen to be collected from the subject of these procedures as well as the requirements applying to the collection activity, the preparation of the subject for this collection. This resource also describes the associated type(s) of specimen conditioned for testing, which are the output of the specimen collection activity. A specimen conditioned for testing is described with the type(s) of container and possible additive to be used, the minimal and normal volumes of collection, the conditions of storage, transportation and handling for the specimen once collected and conditioned. This resource is a necessary building block of a sharable catalog of orderable in vitro diagnostic services. The subjects of these orderable services may be human patients, non-human living subjects or non-living materials such as water, surfaces, medical devices, etc. All sub-specialties of clinical and anatomic pathology laboratories are concerned, as well as all care services prone to order in vitro diagnostic services to those laboratories. A catalog of orderable services generally belongs to a specific laboratory or facility. Nonetheless, the data items used to build this catalog are of universal meaning and interest.
This resource relates to these other resources:
This resource is referenced by ActivityDefinition, CatalogEntry, ObservationDefinition and itself.
This resource does not implement any patterns.
Structure
| Name | Flags | Card. | Type | Description & Constraints |
|---|---|---|---|---|
 | TU | DomainResource | Kind of specimen Elements defined in Ancestors: id, meta, implicitRules, language, text, contained, extension, modifierExtension | |
  | Σ | 0..1 | uri | Logical canonical URL to reference this SpecimenDefinition (globally unique) |
  | Σ | 0..1 | Identifier | Business identifier |
  | Σ | 0..1 | string | Business version of the SpecimenDefinition |
  | Σ | 0..1 | string | Name for this SpecimenDefinition (Human friendly) |
  | Σ | 0..* | canonical(SpecimenDefinition) | Based on FHIR definition of another SpecimenDefinition |
  | Σ | 0..* | uri | Based on external definition |
  | ?!Σ | 1..1 | code | draft | active | retired | unknown PublicationStatus (Required) |
  | ?!Σ | 0..1 | boolean | If this SpecimenDefinition is not for real usage |
  | Σ | 0..1 | Type of subject for specimen collection | |
   | CodeableConcept | |||
  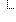 | Reference(Group) | |||
  | Σ | 0..1 | dateTime | Date status first applied |
  | Σ | 0..1 | Reference(Practitioner | PractitionerRole | Organization) | The name of the individual or organization that published the SpecimenDefinition |
  | Σ | 0..* | ContactDetail | Contact details for the publisher |
  | 0..1 | markdown | Natural language description of the SpecimenDefinition | |
  | 0..* | UsageContext | Content intends to support these contexts | |
  | Σ | 0..* | CodeableConcept | Intended jurisdiction for this SpecimenDefinition (if applicable) Jurisdiction (Extensible) |
  | 0..1 | markdown | Why this SpecimenDefinition is defined | |
  | 0..1 | markdown | Use and/or publishing restrictions | |
  | 0..1 | date | When SpecimenDefinition was approved by publisher | |
  | 0..1 | date | Last review date for the SpecimenDefinition | |
  | Σ | 0..1 | Period | The effective date range for the SpecimenDefinition |
  | Σ | 0..1 | CodeableConcept | Kind of material to collect v2 Specimen Type (Example) |
  | Σ | 0..* | CodeableConcept | Patient preparation for collection Patient preparation prior specimen collection (Example) |
  | Σ | 0..1 | string | Time aspect for collection |
  | Σ | 0..* | CodeableConcept | Specimen collection procedure Specimen collection methods (Example) |
  | 0..* | BackboneElement | Specimen in container intended for testing by lab | |
   | 0..1 | boolean | Primary or secondary specimen | |
   | 0..1 | CodeableConcept | Type of intended specimen v2 Specimen Type (Example) | |
   | 1..1 | code | preferred | alternate SpecimenContainedPreference (Required) | |
   | 0..1 | BackboneElement | The specimen's container | |
    | 0..1 | CodeableConcept | Container material Types of material for specimen containers (Example) | |
    | 0..1 | CodeableConcept | Kind of container associated with the kind of specimen Specimen Container Type (Example) | |
    | 0..1 | CodeableConcept | Color of container cap ContainerCap (Example) | |
    | 0..1 | string | Container description | |
    | 0..1 | SimpleQuantity | Container capacity | |
    | 0..1 | Minimum volume | ||
     | SimpleQuantity | |||
    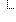 | string | |||
    | 0..* | BackboneElement | Additive associated with container | |
     | 1..1 | Additive associated with container v2 Additive (Example) | ||
      | CodeableConcept | |||
     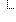 | Reference(Substance) | |||
    | 0..1 | string | Specimen container preparation | |
   | 0..1 | string | Specimen requirements | |
   | 0..1 | Duration | Specimen retention time | |
   | 0..1 | boolean | Specimen for single use only | |
   | 0..* | CodeableConcept | Rejection criterion RejectionCriterion (Example) | |
   | 0..* | BackboneElement | Specimen handling before testing | |
    | 0..1 | CodeableConcept | Temperature qualifier HandlingConditionSet (Example) | |
    | 0..1 | Range | Temperature range | |
    | 0..1 | Duration | Maximum preservation time | |
    | 0..1 | string | Preservation instruction | |
   | 0..* | CodeableConcept | Where the specimen will be tested Diagnostic Service Section Codes (Example) | |
 Documentation for this format Documentation for this format | ||||
UML Diagram (Legend)
XML Template
<SpecimenDefinition xmlns="http://hl7.org/fhir"><!-- from Resource: id, meta, implicitRules, and language --> <!-- from DomainResource: text, contained, extension, and modifierExtension --> <url value="[uri]"/><!-- 0..1 Logical canonical URL to reference this SpecimenDefinition (globally unique) --> <identifier><!-- 0..1 Identifier Business identifier --></identifier> <version value="[string]"/><!-- 0..1 Business version of the SpecimenDefinition --> <title value="[string]"/><!-- 0..1 Name for this SpecimenDefinition (Human friendly) --> <derivedFromCanonical><!-- 0..* canonical(SpecimenDefinition) Based on FHIR definition of another SpecimenDefinition --></derivedFromCanonical> <derivedFromUri value="[uri]"/><!-- 0..* Based on external definition --> <status value="[code]"/><!-- 1..1 draft | active | retired | unknown --> <experimental value="[boolean]"/><!-- 0..1 If this SpecimenDefinition is not for real usage --> <subject[x]><!-- 0..1 CodeableConcept|Reference(Group) Type of subject for specimen collection --></subject[x]> <date value="[dateTime]"/><!-- 0..1 Date status first applied --> <publisher><!-- 0..1 Reference(Organization|Practitioner|PractitionerRole) The name of the individual or organization that published the SpecimenDefinition --></publisher> <contact><!-- 0..* ContactDetail Contact details for the publisher --></contact> <description value="[markdown]"/><!-- 0..1 Natural language description of the SpecimenDefinition --> <useContext><!-- 0..* UsageContext Content intends to support these contexts --></useContext> <jurisdiction><!-- 0..* CodeableConcept Intended jurisdiction for this SpecimenDefinition (if applicable) --></jurisdiction> <purpose value="[markdown]"/><!-- 0..1 Why this SpecimenDefinition is defined --> <copyright value="[markdown]"/><!-- 0..1 Use and/or publishing restrictions --> <approvalDate value="[date]"/><!-- 0..1 When SpecimenDefinition was approved by publisher --> <lastReviewDate value="[date]"/><!-- 0..1 Last review date for the SpecimenDefinition --> <effectivePeriod><!-- 0..1 Period The effective date range for the SpecimenDefinition --></effectivePeriod> <typeCollected><!-- 0..1 CodeableConcept Kind of material to collect --></typeCollected> <patientPreparation><!-- 0..* CodeableConcept Patient preparation for collection --></patientPreparation> <timeAspect value="[string]"/><!-- 0..1 Time aspect for collection --> <collection><!-- 0..* CodeableConcept Specimen collection procedure --></collection> <typeTested> <!-- 0..* Specimen in container intended for testing by lab --> <isDerived value="[boolean]"/><!-- 0..1 Primary or secondary specimen --> <type><!-- 0..1 CodeableConcept Type of intended specimen --></type> <preference value="[code]"/><!-- 1..1 preferred | alternate --> <container> <!-- 0..1 The specimen's container --> <material><!-- 0..1 CodeableConcept Container material --></material> <type><!-- 0..1 CodeableConcept Kind of container associated with the kind of specimen --></type> <cap><!-- 0..1 CodeableConcept Color of container cap --></cap> <description value="[string]"/><!-- 0..1 Container description --> <capacity><!-- 0..1 Quantity(SimpleQuantity) Container capacity --></capacity> <minimumVolume[x]><!-- 0..1 Quantity(SimpleQuantity)|string Minimum volume --></minimumVolume[x]> <additive> <!-- 0..* Additive associated with container --> <additive[x]><!-- 1..1 CodeableConcept|Reference(Substance) Additive associated with container --></additive[x]> </additive> <preparation value="[string]"/><!-- 0..1 Specimen container preparation --> </container> <requirement value="[string]"/><!-- 0..1 Specimen requirements --> <retentionTime><!-- 0..1 Duration Specimen retention time --></retentionTime> <singleUse value="[boolean]"/><!-- 0..1 Specimen for single use only --> <rejectionCriterion><!-- 0..* CodeableConcept Rejection criterion --></rejectionCriterion> <handling> <!-- 0..* Specimen handling before testing --> <temperatureQualifier><!-- 0..1 CodeableConcept Temperature qualifier --></temperatureQualifier> <temperatureRange><!-- 0..1 Range Temperature range --></temperatureRange> <maxDuration><!-- 0..1 Duration Maximum preservation time --></maxDuration> <instruction value="[string]"/><!-- 0..1 Preservation instruction --> </handling> <testingDestination><!-- 0..* CodeableConcept Where the specimen will be tested --></testingDestination> </typeTested> </SpecimenDefinition>
JSON Template
{ "resourceType" : "SpecimenDefinition",
// from Resource: id, meta, implicitRules, and language
// from DomainResource: text, contained, extension, and modifierExtension
"url" : "<uri>", // Logical canonical URL to reference this SpecimenDefinition (globally unique)
"identifier" : { Identifier }, // Business identifier
"version" : "<string>", // Business version of the SpecimenDefinition
"title" : "<string>", // Name for this SpecimenDefinition (Human friendly)
"derivedFromCanonical" : [{ canonical(SpecimenDefinition) }], // Based on FHIR definition of another SpecimenDefinition
"derivedFromUri" : ["<uri>"], // Based on external definition
"status" : "<code>", // R! draft | active | retired | unknown
"experimental" : <boolean>, // If this SpecimenDefinition is not for real usage
// subject[x]: Type of subject for specimen collection. One of these 2:
"subjectCodeableConcept" : { CodeableConcept },
"subjectReference" : { Reference(Group) },
"date" : "<dateTime>", // Date status first applied
"publisher" : { Reference(Organization|Practitioner|PractitionerRole) }, // The name of the individual or organization that published the SpecimenDefinition
"contact" : [{ ContactDetail }], // Contact details for the publisher
"description" : "<markdown>", // Natural language description of the SpecimenDefinition
"useContext" : [{ UsageContext }], // Content intends to support these contexts
"jurisdiction" : [{ CodeableConcept }], // Intended jurisdiction for this SpecimenDefinition (if applicable)
"purpose" : "<markdown>", // Why this SpecimenDefinition is defined
"copyright" : "<markdown>", // Use and/or publishing restrictions
"approvalDate" : "<date>", // When SpecimenDefinition was approved by publisher
"lastReviewDate" : "<date>", // Last review date for the SpecimenDefinition
"effectivePeriod" : { Period }, // The effective date range for the SpecimenDefinition
"typeCollected" : { CodeableConcept }, // Kind of material to collect
"patientPreparation" : [{ CodeableConcept }], // Patient preparation for collection
"timeAspect" : "<string>", // Time aspect for collection
"collection" : [{ CodeableConcept }], // Specimen collection procedure
"typeTested" : [{ // Specimen in container intended for testing by lab
"isDerived" : <boolean>, // Primary or secondary specimen
"type" : { CodeableConcept }, // Type of intended specimen
"preference" : "<code>", // R! preferred | alternate
"container" : { // The specimen's container
"material" : { CodeableConcept }, // Container material
"type" : { CodeableConcept }, // Kind of container associated with the kind of specimen
"cap" : { CodeableConcept }, // Color of container cap
"description" : "<string>", // Container description
"capacity" : { Quantity(SimpleQuantity) }, // Container capacity
// minimumVolume[x]: Minimum volume. One of these 2:
"minimumVolumeQuantity" : { Quantity(SimpleQuantity) },
"minimumVolumeString" : "<string>",
"additive" : [{ // Additive associated with container
// additive[x]: Additive associated with container. One of these 2:
"additiveCodeableConcept" : { CodeableConcept }
"additiveReference" : { Reference(Substance) }
}],
"preparation" : "<string>" // Specimen container preparation
},
"requirement" : "<string>", // Specimen requirements
"retentionTime" : { Duration }, // Specimen retention time
"singleUse" : <boolean>, // Specimen for single use only
"rejectionCriterion" : [{ CodeableConcept }], // Rejection criterion
"handling" : [{ // Specimen handling before testing
"temperatureQualifier" : { CodeableConcept }, // Temperature qualifier
"temperatureRange" : { Range }, // Temperature range
"maxDuration" : { Duration }, // Maximum preservation time
"instruction" : "<string>" // Preservation instruction
}],
"testingDestination" : [{ CodeableConcept }] // Where the specimen will be tested
}]
}
"resourceType" : "SpecimenDefinition",
// from Resource: id, meta, implicitRules, and language
// from DomainResource: text, contained, extension, and modifierExtension
"url" : "<uri>", // Logical canonical URL to reference this SpecimenDefinition (globally unique)
"identifier" : { Identifier }, // Business identifier
"version" : "<string>", // Business version of the SpecimenDefinition
"title" : "<string>", // Name for this SpecimenDefinition (Human friendly)
"derivedFromCanonical" : [{ canonical(SpecimenDefinition) }], // Based on FHIR definition of another SpecimenDefinition
"derivedFromUri" : ["<uri>"], // Based on external definition
"status" : "<code>", // R! draft | active | retired | unknown
"experimental" : <boolean>, // If this SpecimenDefinition is not for real usage
// subject[x]: Type of subject for specimen collection. One of these 2:
"subjectCodeableConcept" : { CodeableConcept },
"subjectReference" : { Reference(Group) },
"date" : "<dateTime>", // Date status first applied
"publisher" : { Reference(Organization|Practitioner|PractitionerRole) }, // The name of the individual or organization that published the SpecimenDefinition
"contact" : [{ ContactDetail }], // Contact details for the publisher
"description" : "<markdown>", // Natural language description of the SpecimenDefinition
"useContext" : [{ UsageContext }], // Content intends to support these contexts
"jurisdiction" : [{ CodeableConcept }], // Intended jurisdiction for this SpecimenDefinition (if applicable)
"purpose" : "<markdown>", // Why this SpecimenDefinition is defined
"copyright" : "<markdown>", // Use and/or publishing restrictions
"approvalDate" : "<date>", // When SpecimenDefinition was approved by publisher
"lastReviewDate" : "<date>", // Last review date for the SpecimenDefinition
"effectivePeriod" : { Period }, // The effective date range for the SpecimenDefinition
"typeCollected" : { CodeableConcept }, // Kind of material to collect
"patientPreparation" : [{ CodeableConcept }], // Patient preparation for collection
"timeAspect" : "<string>", // Time aspect for collection
"collection" : [{ CodeableConcept }], // Specimen collection procedure
"typeTested" : [{ // Specimen in container intended for testing by lab
"isDerived" : <boolean>, // Primary or secondary specimen
"type" : { CodeableConcept }, // Type of intended specimen
"preference" : "<code>", // R! preferred | alternate
"container" : { // The specimen's container
"material" : { CodeableConcept }, // Container material
"type" : { CodeableConcept }, // Kind of container associated with the kind of specimen
"cap" : { CodeableConcept }, // Color of container cap
"description" : "<string>", // Container description
"capacity" : { Quantity(SimpleQuantity) }, // Container capacity
// minimumVolume[x]: Minimum volume. One of these 2:
"minimumVolumeQuantity" : { Quantity(SimpleQuantity) },
"minimumVolumeString" : "<string>",
"additive" : [{ // Additive associated with container
// additive[x]: Additive associated with container. One of these 2:
"additiveCodeableConcept" : { CodeableConcept }
"additiveReference" : { Reference(Substance) }
}],
"preparation" : "<string>" // Specimen container preparation
},
"requirement" : "<string>", // Specimen requirements
"retentionTime" : { Duration }, // Specimen retention time
"singleUse" : <boolean>, // Specimen for single use only
"rejectionCriterion" : [{ CodeableConcept }], // Rejection criterion
"handling" : [{ // Specimen handling before testing
"temperatureQualifier" : { CodeableConcept }, // Temperature qualifier
"temperatureRange" : { Range }, // Temperature range
"maxDuration" : { Duration }, // Maximum preservation time
"instruction" : "<string>" // Preservation instruction
}],
"testingDestination" : [{ CodeableConcept }] // Where the specimen will be tested
}]
}
Turtle Template
@prefix fhir: <http://hl7.org/fhir/> .[ a fhir:SpecimenDefinition; fhir:nodeRole fhir:treeRoot; # if this is the parser root # from Resource: .id, .meta, .implicitRules, and .language # from DomainResource: .text, .contained, .extension, and .modifierExtension fhir:SpecimenDefinition.url [ uri ]; # 0..1 Logical canonical URL to reference this SpecimenDefinition (globally unique) fhir:SpecimenDefinition.identifier [ Identifier ]; # 0..1 Business identifier fhir:SpecimenDefinition.version [ string ]; # 0..1 Business version of the SpecimenDefinition fhir:SpecimenDefinition.title [ string ]; # 0..1 Name for this SpecimenDefinition (Human friendly) fhir:SpecimenDefinition.derivedFromCanonical [ canonical(SpecimenDefinition) ], ... ; # 0..* Based on FHIR definition of another SpecimenDefinition fhir:SpecimenDefinition.derivedFromUri [ uri ], ... ; # 0..* Based on external definition fhir:SpecimenDefinition.status [ code ]; # 1..1 draft | active | retired | unknown fhir:SpecimenDefinition.experimental [ boolean ]; # 0..1 If this SpecimenDefinition is not for real usage # SpecimenDefinition.subject[x] : 0..1 Type of subject for specimen collection. One of these 2 fhir:SpecimenDefinition.subjectCodeableConcept [ CodeableConcept ] fhir:SpecimenDefinition.subjectReference [ Reference(Group) ] fhir:SpecimenDefinition.date [ dateTime ]; # 0..1 Date status first applied fhir:SpecimenDefinition.publisher [ Reference(Organization|Practitioner|PractitionerRole) ]; # 0..1 The name of the individual or organization that published the SpecimenDefinition fhir:SpecimenDefinition.contact [ ContactDetail ], ... ; # 0..* Contact details for the publisher fhir:SpecimenDefinition.description [ markdown ]; # 0..1 Natural language description of the SpecimenDefinition fhir:SpecimenDefinition.useContext [ UsageContext ], ... ; # 0..* Content intends to support these contexts fhir:SpecimenDefinition.jurisdiction [ CodeableConcept ], ... ; # 0..* Intended jurisdiction for this SpecimenDefinition (if applicable) fhir:SpecimenDefinition.purpose [ markdown ]; # 0..1 Why this SpecimenDefinition is defined fhir:SpecimenDefinition.copyright [ markdown ]; # 0..1 Use and/or publishing restrictions fhir:SpecimenDefinition.approvalDate [ date ]; # 0..1 When SpecimenDefinition was approved by publisher fhir:SpecimenDefinition.lastReviewDate [ date ]; # 0..1 Last review date for the SpecimenDefinition fhir:SpecimenDefinition.effectivePeriod [ Period ]; # 0..1 The effective date range for the SpecimenDefinition fhir:SpecimenDefinition.typeCollected [ CodeableConcept ]; # 0..1 Kind of material to collect fhir:SpecimenDefinition.patientPreparation [ CodeableConcept ], ... ; # 0..* Patient preparation for collection fhir:SpecimenDefinition.timeAspect [ string ]; # 0..1 Time aspect for collection fhir:SpecimenDefinition.collection [ CodeableConcept ], ... ; # 0..* Specimen collection procedure fhir:SpecimenDefinition.typeTested [ # 0..* Specimen in container intended for testing by lab fhir:SpecimenDefinition.typeTested.isDerived [ boolean ]; # 0..1 Primary or secondary specimen fhir:SpecimenDefinition.typeTested.type [ CodeableConcept ]; # 0..1 Type of intended specimen fhir:SpecimenDefinition.typeTested.preference [ code ]; # 1..1 preferred | alternate fhir:SpecimenDefinition.typeTested.container [ # 0..1 The specimen's container fhir:SpecimenDefinition.typeTested.container.material [ CodeableConcept ]; # 0..1 Container material fhir:SpecimenDefinition.typeTested.container.type [ CodeableConcept ]; # 0..1 Kind of container associated with the kind of specimen fhir:SpecimenDefinition.typeTested.container.cap [ CodeableConcept ]; # 0..1 Color of container cap fhir:SpecimenDefinition.typeTested.container.description [ string ]; # 0..1 Container description fhir:SpecimenDefinition.typeTested.container.capacity [ Quantity(SimpleQuantity) ]; # 0..1 Container capacity # SpecimenDefinition.typeTested.container.minimumVolume[x] : 0..1 Minimum volume. One of these 2 fhir:SpecimenDefinition.typeTested.container.minimumVolumeSimpleQuantity [ Quantity(SimpleQuantity) ] fhir:SpecimenDefinition.typeTested.container.minimumVolumeString [ string ] fhir:SpecimenDefinition.typeTested.container.additive [ # 0..* Additive associated with container # SpecimenDefinition.typeTested.container.additive.additive[x] : 1..1 Additive associated with container. One of these 2 fhir:SpecimenDefinition.typeTested.container.additive.additiveCodeableConcept [ CodeableConcept ] fhir:SpecimenDefinition.typeTested.container.additive.additiveReference [ Reference(Substance) ] ], ...; fhir:SpecimenDefinition.typeTested.container.preparation [ string ]; # 0..1 Specimen container preparation ]; fhir:SpecimenDefinition.typeTested.requirement [ string ]; # 0..1 Specimen requirements fhir:SpecimenDefinition.typeTested.retentionTime [ Duration ]; # 0..1 Specimen retention time fhir:SpecimenDefinition.typeTested.singleUse [ boolean ]; # 0..1 Specimen for single use only fhir:SpecimenDefinition.typeTested.rejectionCriterion [ CodeableConcept ], ... ; # 0..* Rejection criterion fhir:SpecimenDefinition.typeTested.handling [ # 0..* Specimen handling before testing fhir:SpecimenDefinition.typeTested.handling.temperatureQualifier [ CodeableConcept ]; # 0..1 Temperature qualifier fhir:SpecimenDefinition.typeTested.handling.temperatureRange [ Range ]; # 0..1 Temperature range fhir:SpecimenDefinition.typeTested.handling.maxDuration [ Duration ]; # 0..1 Maximum preservation time fhir:SpecimenDefinition.typeTested.handling.instruction [ string ]; # 0..1 Preservation instruction ], ...; fhir:SpecimenDefinition.typeTested.testingDestination [ CodeableConcept ], ... ; # 0..* Where the specimen will be tested ], ...; ]
Changes since R3
| SpecimenDefinition | |
| SpecimenDefinition.url |
|
| SpecimenDefinition.version |
|
| SpecimenDefinition.title |
|
| SpecimenDefinition.derivedFromCanonical |
|
| SpecimenDefinition.derivedFromUri |
|
| SpecimenDefinition.status |
|
| SpecimenDefinition.experimental |
|
| SpecimenDefinition.subject[x] |
|
| SpecimenDefinition.date |
|
| SpecimenDefinition.publisher |
|
| SpecimenDefinition.contact |
|
| SpecimenDefinition.description |
|
| SpecimenDefinition.useContext |
|
| SpecimenDefinition.jurisdiction |
|
| SpecimenDefinition.purpose |
|
| SpecimenDefinition.copyright |
|
| SpecimenDefinition.approvalDate |
|
| SpecimenDefinition.lastReviewDate |
|
| SpecimenDefinition.effectivePeriod |
|
| SpecimenDefinition.typeTested.preference |
|
| SpecimenDefinition.typeTested.container.minimumVolume[x] |
|
| SpecimenDefinition.typeTested.singleUse |
|
| SpecimenDefinition.typeTested.testingDestination |
|
See the Full Difference for further information
This analysis is available as XML or JSON.
See R3 <--> R4 Conversion Maps (status = Not Mapped)
Structure
| Name | Flags | Card. | Type | Description & Constraints |
|---|---|---|---|---|
 | TU | DomainResource | Kind of specimen Elements defined in Ancestors: id, meta, implicitRules, language, text, contained, extension, modifierExtension | |
  | Σ | 0..1 | uri | Logical canonical URL to reference this SpecimenDefinition (globally unique) |
  | Σ | 0..1 | Identifier | Business identifier |
  | Σ | 0..1 | string | Business version of the SpecimenDefinition |
  | Σ | 0..1 | string | Name for this SpecimenDefinition (Human friendly) |
  | Σ | 0..* | canonical(SpecimenDefinition) | Based on FHIR definition of another SpecimenDefinition |
  | Σ | 0..* | uri | Based on external definition |
  | ?!Σ | 1..1 | code | draft | active | retired | unknown PublicationStatus (Required) |
  | ?!Σ | 0..1 | boolean | If this SpecimenDefinition is not for real usage |
  | Σ | 0..1 | Type of subject for specimen collection | |
   | CodeableConcept | |||
   | Reference(Group) | |||
  | Σ | 0..1 | dateTime | Date status first applied |
  | Σ | 0..1 | Reference(Practitioner | PractitionerRole | Organization) | The name of the individual or organization that published the SpecimenDefinition |
  | Σ | 0..* | ContactDetail | Contact details for the publisher |
  | 0..1 | markdown | Natural language description of the SpecimenDefinition | |
  | 0..* | UsageContext | Content intends to support these contexts | |
  | Σ | 0..* | CodeableConcept | Intended jurisdiction for this SpecimenDefinition (if applicable) Jurisdiction (Extensible) |
  | 0..1 | markdown | Why this SpecimenDefinition is defined | |
  | 0..1 | markdown | Use and/or publishing restrictions | |
  | 0..1 | date | When SpecimenDefinition was approved by publisher | |
  | 0..1 | date | Last review date for the SpecimenDefinition | |
  | Σ | 0..1 | Period | The effective date range for the SpecimenDefinition |
  | Σ | 0..1 | CodeableConcept | Kind of material to collect v2 Specimen Type (Example) |
  | Σ | 0..* | CodeableConcept | Patient preparation for collection Patient preparation prior specimen collection (Example) |
  | Σ | 0..1 | string | Time aspect for collection |
  | Σ | 0..* | CodeableConcept | Specimen collection procedure Specimen collection methods (Example) |
  | 0..* | BackboneElement | Specimen in container intended for testing by lab | |
   | 0..1 | boolean | Primary or secondary specimen | |
   | 0..1 | CodeableConcept | Type of intended specimen v2 Specimen Type (Example) | |
   | 1..1 | code | preferred | alternate SpecimenContainedPreference (Required) | |
   | 0..1 | BackboneElement | The specimen's container | |
    | 0..1 | CodeableConcept | Container material Types of material for specimen containers (Example) | |
    | 0..1 | CodeableConcept | Kind of container associated with the kind of specimen Specimen Container Type (Example) | |
    | 0..1 | CodeableConcept | Color of container cap ContainerCap (Example) | |
    | 0..1 | string | Container description | |
    | 0..1 | SimpleQuantity | Container capacity | |
    | 0..1 | Minimum volume | ||
     | SimpleQuantity | |||
     | string | |||
    | 0..* | BackboneElement | Additive associated with container | |
     | 1..1 | Additive associated with container v2 Additive (Example) | ||
      | CodeableConcept | |||
      | Reference(Substance) | |||
    | 0..1 | string | Specimen container preparation | |
   | 0..1 | string | Specimen requirements | |
   | 0..1 | Duration | Specimen retention time | |
   | 0..1 | boolean | Specimen for single use only | |
   | 0..* | CodeableConcept | Rejection criterion RejectionCriterion (Example) | |
   | 0..* | BackboneElement | Specimen handling before testing | |
    | 0..1 | CodeableConcept | Temperature qualifier HandlingConditionSet (Example) | |
    | 0..1 | Range | Temperature range | |
    | 0..1 | Duration | Maximum preservation time | |
    | 0..1 | string | Preservation instruction | |
   | 0..* | CodeableConcept | Where the specimen will be tested Diagnostic Service Section Codes (Example) | |
 Documentation for this format Documentation for this format | ||||
XML Template
<SpecimenDefinition xmlns="http://hl7.org/fhir"><!-- from Resource: id, meta, implicitRules, and language --> <!-- from DomainResource: text, contained, extension, and modifierExtension --> <url value="[uri]"/><!-- 0..1 Logical canonical URL to reference this SpecimenDefinition (globally unique) --> <identifier><!-- 0..1 Identifier Business identifier --></identifier> <version value="[string]"/><!-- 0..1 Business version of the SpecimenDefinition --> <title value="[string]"/><!-- 0..1 Name for this SpecimenDefinition (Human friendly) --> <derivedFromCanonical><!-- 0..* canonical(SpecimenDefinition) Based on FHIR definition of another SpecimenDefinition --></derivedFromCanonical> <derivedFromUri value="[uri]"/><!-- 0..* Based on external definition --> <status value="[code]"/><!-- 1..1 draft | active | retired | unknown --> <experimental value="[boolean]"/><!-- 0..1 If this SpecimenDefinition is not for real usage --> <subject[x]><!-- 0..1 CodeableConcept|Reference(Group) Type of subject for specimen collection --></subject[x]> <date value="[dateTime]"/><!-- 0..1 Date status first applied --> <publisher><!-- 0..1 Reference(Organization|Practitioner|PractitionerRole) The name of the individual or organization that published the SpecimenDefinition --></publisher> <contact><!-- 0..* ContactDetail Contact details for the publisher --></contact> <description value="[markdown]"/><!-- 0..1 Natural language description of the SpecimenDefinition --> <useContext><!-- 0..* UsageContext Content intends to support these contexts --></useContext> <jurisdiction><!-- 0..* CodeableConcept Intended jurisdiction for this SpecimenDefinition (if applicable) --></jurisdiction> <purpose value="[markdown]"/><!-- 0..1 Why this SpecimenDefinition is defined --> <copyright value="[markdown]"/><!-- 0..1 Use and/or publishing restrictions --> <approvalDate value="[date]"/><!-- 0..1 When SpecimenDefinition was approved by publisher --> <lastReviewDate value="[date]"/><!-- 0..1 Last review date for the SpecimenDefinition --> <effectivePeriod><!-- 0..1 Period The effective date range for the SpecimenDefinition --></effectivePeriod> <typeCollected><!-- 0..1 CodeableConcept Kind of material to collect --></typeCollected> <patientPreparation><!-- 0..* CodeableConcept Patient preparation for collection --></patientPreparation> <timeAspect value="[string]"/><!-- 0..1 Time aspect for collection --> <collection><!-- 0..* CodeableConcept Specimen collection procedure --></collection> <typeTested> <!-- 0..* Specimen in container intended for testing by lab --> <isDerived value="[boolean]"/><!-- 0..1 Primary or secondary specimen --> <type><!-- 0..1 CodeableConcept Type of intended specimen --></type> <preference value="[code]"/><!-- 1..1 preferred | alternate --> <container> <!-- 0..1 The specimen's container --> <material><!-- 0..1 CodeableConcept Container material --></material> <type><!-- 0..1 CodeableConcept Kind of container associated with the kind of specimen --></type> <cap><!-- 0..1 CodeableConcept Color of container cap --></cap> <description value="[string]"/><!-- 0..1 Container description --> <capacity><!-- 0..1 Quantity(SimpleQuantity) Container capacity --></capacity> <minimumVolume[x]><!-- 0..1 Quantity(SimpleQuantity)|string Minimum volume --></minimumVolume[x]> <additive> <!-- 0..* Additive associated with container --> <additive[x]><!-- 1..1 CodeableConcept|Reference(Substance) Additive associated with container --></additive[x]> </additive> <preparation value="[string]"/><!-- 0..1 Specimen container preparation --> </container> <requirement value="[string]"/><!-- 0..1 Specimen requirements --> <retentionTime><!-- 0..1 Duration Specimen retention time --></retentionTime> <singleUse value="[boolean]"/><!-- 0..1 Specimen for single use only --> <rejectionCriterion><!-- 0..* CodeableConcept Rejection criterion --></rejectionCriterion> <handling> <!-- 0..* Specimen handling before testing --> <temperatureQualifier><!-- 0..1 CodeableConcept Temperature qualifier --></temperatureQualifier> <temperatureRange><!-- 0..1 Range Temperature range --></temperatureRange> <maxDuration><!-- 0..1 Duration Maximum preservation time --></maxDuration> <instruction value="[string]"/><!-- 0..1 Preservation instruction --> </handling> <testingDestination><!-- 0..* CodeableConcept Where the specimen will be tested --></testingDestination> </typeTested> </SpecimenDefinition>
JSON Template
{ "resourceType" : "SpecimenDefinition",
// from Resource: id, meta, implicitRules, and language
// from DomainResource: text, contained, extension, and modifierExtension
"url" : "<uri>", // Logical canonical URL to reference this SpecimenDefinition (globally unique)
"identifier" : { Identifier }, // Business identifier
"version" : "<string>", // Business version of the SpecimenDefinition
"title" : "<string>", // Name for this SpecimenDefinition (Human friendly)
"derivedFromCanonical" : [{ canonical(SpecimenDefinition) }], // Based on FHIR definition of another SpecimenDefinition
"derivedFromUri" : ["<uri>"], // Based on external definition
"status" : "<code>", // R! draft | active | retired | unknown
"experimental" : <boolean>, // If this SpecimenDefinition is not for real usage
// subject[x]: Type of subject for specimen collection. One of these 2:
"subjectCodeableConcept" : { CodeableConcept },
"subjectReference" : { Reference(Group) },
"date" : "<dateTime>", // Date status first applied
"publisher" : { Reference(Organization|Practitioner|PractitionerRole) }, // The name of the individual or organization that published the SpecimenDefinition
"contact" : [{ ContactDetail }], // Contact details for the publisher
"description" : "<markdown>", // Natural language description of the SpecimenDefinition
"useContext" : [{ UsageContext }], // Content intends to support these contexts
"jurisdiction" : [{ CodeableConcept }], // Intended jurisdiction for this SpecimenDefinition (if applicable)
"purpose" : "<markdown>", // Why this SpecimenDefinition is defined
"copyright" : "<markdown>", // Use and/or publishing restrictions
"approvalDate" : "<date>", // When SpecimenDefinition was approved by publisher
"lastReviewDate" : "<date>", // Last review date for the SpecimenDefinition
"effectivePeriod" : { Period }, // The effective date range for the SpecimenDefinition
"typeCollected" : { CodeableConcept }, // Kind of material to collect
"patientPreparation" : [{ CodeableConcept }], // Patient preparation for collection
"timeAspect" : "<string>", // Time aspect for collection
"collection" : [{ CodeableConcept }], // Specimen collection procedure
"typeTested" : [{ // Specimen in container intended for testing by lab
"isDerived" : <boolean>, // Primary or secondary specimen
"type" : { CodeableConcept }, // Type of intended specimen
"preference" : "<code>", // R! preferred | alternate
"container" : { // The specimen's container
"material" : { CodeableConcept }, // Container material
"type" : { CodeableConcept }, // Kind of container associated with the kind of specimen
"cap" : { CodeableConcept }, // Color of container cap
"description" : "<string>", // Container description
"capacity" : { Quantity(SimpleQuantity) }, // Container capacity
// minimumVolume[x]: Minimum volume. One of these 2:
"minimumVolumeQuantity" : { Quantity(SimpleQuantity) },
"minimumVolumeString" : "<string>",
"additive" : [{ // Additive associated with container
// additive[x]: Additive associated with container. One of these 2:
"additiveCodeableConcept" : { CodeableConcept }
"additiveReference" : { Reference(Substance) }
}],
"preparation" : "<string>" // Specimen container preparation
},
"requirement" : "<string>", // Specimen requirements
"retentionTime" : { Duration }, // Specimen retention time
"singleUse" : <boolean>, // Specimen for single use only
"rejectionCriterion" : [{ CodeableConcept }], // Rejection criterion
"handling" : [{ // Specimen handling before testing
"temperatureQualifier" : { CodeableConcept }, // Temperature qualifier
"temperatureRange" : { Range }, // Temperature range
"maxDuration" : { Duration }, // Maximum preservation time
"instruction" : "<string>" // Preservation instruction
}],
"testingDestination" : [{ CodeableConcept }] // Where the specimen will be tested
}]
}
"resourceType" : "SpecimenDefinition",
// from Resource: id, meta, implicitRules, and language
// from DomainResource: text, contained, extension, and modifierExtension
"url" : "<uri>", // Logical canonical URL to reference this SpecimenDefinition (globally unique)
"identifier" : { Identifier }, // Business identifier
"version" : "<string>", // Business version of the SpecimenDefinition
"title" : "<string>", // Name for this SpecimenDefinition (Human friendly)
"derivedFromCanonical" : [{ canonical(SpecimenDefinition) }], // Based on FHIR definition of another SpecimenDefinition
"derivedFromUri" : ["<uri>"], // Based on external definition
"status" : "<code>", // R! draft | active | retired | unknown
"experimental" : <boolean>, // If this SpecimenDefinition is not for real usage
// subject[x]: Type of subject for specimen collection. One of these 2:
"subjectCodeableConcept" : { CodeableConcept },
"subjectReference" : { Reference(Group) },
"date" : "<dateTime>", // Date status first applied
"publisher" : { Reference(Organization|Practitioner|PractitionerRole) }, // The name of the individual or organization that published the SpecimenDefinition
"contact" : [{ ContactDetail }], // Contact details for the publisher
"description" : "<markdown>", // Natural language description of the SpecimenDefinition
"useContext" : [{ UsageContext }], // Content intends to support these contexts
"jurisdiction" : [{ CodeableConcept }], // Intended jurisdiction for this SpecimenDefinition (if applicable)
"purpose" : "<markdown>", // Why this SpecimenDefinition is defined
"copyright" : "<markdown>", // Use and/or publishing restrictions
"approvalDate" : "<date>", // When SpecimenDefinition was approved by publisher
"lastReviewDate" : "<date>", // Last review date for the SpecimenDefinition
"effectivePeriod" : { Period }, // The effective date range for the SpecimenDefinition
"typeCollected" : { CodeableConcept }, // Kind of material to collect
"patientPreparation" : [{ CodeableConcept }], // Patient preparation for collection
"timeAspect" : "<string>", // Time aspect for collection
"collection" : [{ CodeableConcept }], // Specimen collection procedure
"typeTested" : [{ // Specimen in container intended for testing by lab
"isDerived" : <boolean>, // Primary or secondary specimen
"type" : { CodeableConcept }, // Type of intended specimen
"preference" : "<code>", // R! preferred | alternate
"container" : { // The specimen's container
"material" : { CodeableConcept }, // Container material
"type" : { CodeableConcept }, // Kind of container associated with the kind of specimen
"cap" : { CodeableConcept }, // Color of container cap
"description" : "<string>", // Container description
"capacity" : { Quantity(SimpleQuantity) }, // Container capacity
// minimumVolume[x]: Minimum volume. One of these 2:
"minimumVolumeQuantity" : { Quantity(SimpleQuantity) },
"minimumVolumeString" : "<string>",
"additive" : [{ // Additive associated with container
// additive[x]: Additive associated with container. One of these 2:
"additiveCodeableConcept" : { CodeableConcept }
"additiveReference" : { Reference(Substance) }
}],
"preparation" : "<string>" // Specimen container preparation
},
"requirement" : "<string>", // Specimen requirements
"retentionTime" : { Duration }, // Specimen retention time
"singleUse" : <boolean>, // Specimen for single use only
"rejectionCriterion" : [{ CodeableConcept }], // Rejection criterion
"handling" : [{ // Specimen handling before testing
"temperatureQualifier" : { CodeableConcept }, // Temperature qualifier
"temperatureRange" : { Range }, // Temperature range
"maxDuration" : { Duration }, // Maximum preservation time
"instruction" : "<string>" // Preservation instruction
}],
"testingDestination" : [{ CodeableConcept }] // Where the specimen will be tested
}]
}
Turtle Template
@prefix fhir: <http://hl7.org/fhir/> .[ a fhir:SpecimenDefinition; fhir:nodeRole fhir:treeRoot; # if this is the parser root # from Resource: .id, .meta, .implicitRules, and .language # from DomainResource: .text, .contained, .extension, and .modifierExtension fhir:SpecimenDefinition.url [ uri ]; # 0..1 Logical canonical URL to reference this SpecimenDefinition (globally unique) fhir:SpecimenDefinition.identifier [ Identifier ]; # 0..1 Business identifier fhir:SpecimenDefinition.version [ string ]; # 0..1 Business version of the SpecimenDefinition fhir:SpecimenDefinition.title [ string ]; # 0..1 Name for this SpecimenDefinition (Human friendly) fhir:SpecimenDefinition.derivedFromCanonical [ canonical(SpecimenDefinition) ], ... ; # 0..* Based on FHIR definition of another SpecimenDefinition fhir:SpecimenDefinition.derivedFromUri [ uri ], ... ; # 0..* Based on external definition fhir:SpecimenDefinition.status [ code ]; # 1..1 draft | active | retired | unknown fhir:SpecimenDefinition.experimental [ boolean ]; # 0..1 If this SpecimenDefinition is not for real usage # SpecimenDefinition.subject[x] : 0..1 Type of subject for specimen collection. One of these 2 fhir:SpecimenDefinition.subjectCodeableConcept [ CodeableConcept ] fhir:SpecimenDefinition.subjectReference [ Reference(Group) ] fhir:SpecimenDefinition.date [ dateTime ]; # 0..1 Date status first applied fhir:SpecimenDefinition.publisher [ Reference(Organization|Practitioner|PractitionerRole) ]; # 0..1 The name of the individual or organization that published the SpecimenDefinition fhir:SpecimenDefinition.contact [ ContactDetail ], ... ; # 0..* Contact details for the publisher fhir:SpecimenDefinition.description [ markdown ]; # 0..1 Natural language description of the SpecimenDefinition fhir:SpecimenDefinition.useContext [ UsageContext ], ... ; # 0..* Content intends to support these contexts fhir:SpecimenDefinition.jurisdiction [ CodeableConcept ], ... ; # 0..* Intended jurisdiction for this SpecimenDefinition (if applicable) fhir:SpecimenDefinition.purpose [ markdown ]; # 0..1 Why this SpecimenDefinition is defined fhir:SpecimenDefinition.copyright [ markdown ]; # 0..1 Use and/or publishing restrictions fhir:SpecimenDefinition.approvalDate [ date ]; # 0..1 When SpecimenDefinition was approved by publisher fhir:SpecimenDefinition.lastReviewDate [ date ]; # 0..1 Last review date for the SpecimenDefinition fhir:SpecimenDefinition.effectivePeriod [ Period ]; # 0..1 The effective date range for the SpecimenDefinition fhir:SpecimenDefinition.typeCollected [ CodeableConcept ]; # 0..1 Kind of material to collect fhir:SpecimenDefinition.patientPreparation [ CodeableConcept ], ... ; # 0..* Patient preparation for collection fhir:SpecimenDefinition.timeAspect [ string ]; # 0..1 Time aspect for collection fhir:SpecimenDefinition.collection [ CodeableConcept ], ... ; # 0..* Specimen collection procedure fhir:SpecimenDefinition.typeTested [ # 0..* Specimen in container intended for testing by lab fhir:SpecimenDefinition.typeTested.isDerived [ boolean ]; # 0..1 Primary or secondary specimen fhir:SpecimenDefinition.typeTested.type [ CodeableConcept ]; # 0..1 Type of intended specimen fhir:SpecimenDefinition.typeTested.preference [ code ]; # 1..1 preferred | alternate fhir:SpecimenDefinition.typeTested.container [ # 0..1 The specimen's container fhir:SpecimenDefinition.typeTested.container.material [ CodeableConcept ]; # 0..1 Container material fhir:SpecimenDefinition.typeTested.container.type [ CodeableConcept ]; # 0..1 Kind of container associated with the kind of specimen fhir:SpecimenDefinition.typeTested.container.cap [ CodeableConcept ]; # 0..1 Color of container cap fhir:SpecimenDefinition.typeTested.container.description [ string ]; # 0..1 Container description fhir:SpecimenDefinition.typeTested.container.capacity [ Quantity(SimpleQuantity) ]; # 0..1 Container capacity # SpecimenDefinition.typeTested.container.minimumVolume[x] : 0..1 Minimum volume. One of these 2 fhir:SpecimenDefinition.typeTested.container.minimumVolumeSimpleQuantity [ Quantity(SimpleQuantity) ] fhir:SpecimenDefinition.typeTested.container.minimumVolumeString [ string ] fhir:SpecimenDefinition.typeTested.container.additive [ # 0..* Additive associated with container # SpecimenDefinition.typeTested.container.additive.additive[x] : 1..1 Additive associated with container. One of these 2 fhir:SpecimenDefinition.typeTested.container.additive.additiveCodeableConcept [ CodeableConcept ] fhir:SpecimenDefinition.typeTested.container.additive.additiveReference [ Reference(Substance) ] ], ...; fhir:SpecimenDefinition.typeTested.container.preparation [ string ]; # 0..1 Specimen container preparation ]; fhir:SpecimenDefinition.typeTested.requirement [ string ]; # 0..1 Specimen requirements fhir:SpecimenDefinition.typeTested.retentionTime [ Duration ]; # 0..1 Specimen retention time fhir:SpecimenDefinition.typeTested.singleUse [ boolean ]; # 0..1 Specimen for single use only fhir:SpecimenDefinition.typeTested.rejectionCriterion [ CodeableConcept ], ... ; # 0..* Rejection criterion fhir:SpecimenDefinition.typeTested.handling [ # 0..* Specimen handling before testing fhir:SpecimenDefinition.typeTested.handling.temperatureQualifier [ CodeableConcept ]; # 0..1 Temperature qualifier fhir:SpecimenDefinition.typeTested.handling.temperatureRange [ Range ]; # 0..1 Temperature range fhir:SpecimenDefinition.typeTested.handling.maxDuration [ Duration ]; # 0..1 Maximum preservation time fhir:SpecimenDefinition.typeTested.handling.instruction [ string ]; # 0..1 Preservation instruction ], ...; fhir:SpecimenDefinition.typeTested.testingDestination [ CodeableConcept ], ... ; # 0..* Where the specimen will be tested ], ...; ]
Changes since Release 3
| SpecimenDefinition | |
| SpecimenDefinition.url |
|
| SpecimenDefinition.version |
|
| SpecimenDefinition.title |
|
| SpecimenDefinition.derivedFromCanonical |
|
| SpecimenDefinition.derivedFromUri |
|
| SpecimenDefinition.status |
|
| SpecimenDefinition.experimental |
|
| SpecimenDefinition.subject[x] |
|
| SpecimenDefinition.date |
|
| SpecimenDefinition.publisher |
|
| SpecimenDefinition.contact |
|
| SpecimenDefinition.description |
|
| SpecimenDefinition.useContext |
|
| SpecimenDefinition.jurisdiction |
|
| SpecimenDefinition.purpose |
|
| SpecimenDefinition.copyright |
|
| SpecimenDefinition.approvalDate |
|
| SpecimenDefinition.lastReviewDate |
|
| SpecimenDefinition.effectivePeriod |
|
| SpecimenDefinition.typeTested.preference |
|
| SpecimenDefinition.typeTested.container.minimumVolume[x] |
|
| SpecimenDefinition.typeTested.singleUse |
|
| SpecimenDefinition.typeTested.testingDestination |
|
See the Full Difference for further information
This analysis is available as XML or JSON.
See R3 <--> R4 Conversion Maps (status = Not Mapped)
See the Profiles & Extensions and the alternate definitions: Master Definition XML + JSON, XML Schema/Schematron + JSON Schema, ShEx (for Turtle) + see the extensions, the spreadsheet version & the dependency analysis a
| Path | Definition | Type | Reference |
|---|---|---|---|
| SpecimenDefinition.status | Codes identifying the status of a SpecimenDefinition resource. | Required | PublicationStatus |
| SpecimenDefinition.jurisdiction | Codes for country, country subdivision and region for indicating where a resource is intended to be used. | Extensible | Jurisdiction ValueSet |
| SpecimenDefinition.typeCollected | The type of the specimen to be collected. | Example | v2.0487 |
| SpecimenDefinition.patientPreparation | Checks on the patient prior specimen collection. | Example | PreparePatient |
| SpecimenDefinition.collection | The action to collect a type of specimen. | Example | SpecimenCollection |
| SpecimenDefinition.typeTested.type | The type of specimen conditioned in a container for lab testing. | Example | v2.0487 |
| SpecimenDefinition.typeTested.preference | Degree of preference of a type of conditioned specimen. | Required | SpecimenContainedPreference |
| SpecimenDefinition.typeTested.container.material | Types of material for specimen containers. | Example | ContainerMaterials |
| SpecimenDefinition.typeTested.container.type | Type of specimen container. | Example | SpecimenContainerType |
| SpecimenDefinition.typeTested.container.cap | Color of the container cap. | Example | ContainerCap |
| SpecimenDefinition.typeTested.container.additive.additive[x] | Substance added to specimen container. | Example | v2.0371 |
| SpecimenDefinition.typeTested.rejectionCriterion | Criterion for rejection of the specimen by laboratory. | Example | RejectionCriterion |
| SpecimenDefinition.typeTested.handling.temperatureQualifier | Set of handling instructions prior testing of the specimen. | Example | HandlingConditionSet |
| SpecimenDefinition.typeTested.testingDestination | Codes specifying where the specimen will be tested. | Example | DiagnosticServiceSectionCodes |
Search parameters for this resource. The common parameters also apply. See Searching for more information about searching in REST, messaging, and services.
| Name | Type | Description | Expression | In Common |
| container | token | The type of specimen conditioned in container expected by the lab | SpecimenDefinition.typeTested.container.type | |
| experimental N | token | Not for genuine usage (true) | SpecimenDefinition.experimental | |
| identifier | token | The unique identifier associated with the SpecimenDefinition | SpecimenDefinition.identifier | |
| is-derived N | token | Primary specimen (false) or derived specimen (true) | SpecimenDefinition.typeTested.isDerived | |
| status N | token | Publication status of the SpecimenDefinition: draft, active, retired, unknown | SpecimenDefinition.status | |
| title N | string | Human-friendly name of the SpecimenDefinition | SpecimenDefinition.title | |
| type | token | The type of collected specimen | SpecimenDefinition.typeCollected | |
| type-tested | token | The type of specimen conditioned for testing | SpecimenDefinition.typeTested.type |