This page is part of the Application Data Exchange Assessment Framework and Functional Requirements for Mobile Health (v0.1.0: STU 1 Ballot 1) based on FHIR (HL7® FHIR® Standard) R4. No current official version has been published yet. For a full list of available versions, see the Directory of published versions
Defines constraints on the Device Resource for data communicating about the source of device data.
The official URL for this profile is:
http://hl7.org/fhir/uv/mhealth-framework/StructureDefinition/mhealth-ade-device
Description of Profiles, Differentials, Snapshots and how the different presentations work.
This structure is derived from Device
This structure is derived from Device
| Name | Flags | Card. | Type | Description & Constraints |
|---|---|---|---|---|
 | 0..* | Device | Item used in healthcare | |
 Documentation for this format Documentation for this format | ||||
| Name | Flags | Card. | Type | Description & Constraints |
|---|---|---|---|---|
 | I | 0..* | Device | Item used in healthcare |
  | Σ | 0..1 | string | Logical id of this artifact |
  | ΣI | 0..1 | Meta | Metadata about the resource |
  | ?!ΣI | 0..1 | uri | A set of rules under which this content was created |
  | I | 0..1 | code | Language of the resource content Binding: CommonLanguages (preferred) Max Binding: AllLanguages |
  | I | 0..1 | Narrative | Text summary of the resource, for human interpretation |
  | 0..* | Resource | Contained, inline Resources | |
  | I | 0..* | Extension | Additional content defined by implementations |
  | ?!I | 0..* | Extension | Extensions that cannot be ignored |
  | I | 0..* | Identifier | Instance identifier |
  | I | 0..1 | Reference(DeviceDefinition) | The reference to the definition for the device |
  | ΣI | 0..* | BackboneElement | Unique Device Identifier (UDI) Barcode string |
   | 0..1 | string | Unique id for inter-element referencing | |
   | I | 0..* | Extension | Additional content defined by implementations |
   | ?!ΣI | 0..* | Extension | Extensions that cannot be ignored even if unrecognized |
   | ΣI | 0..1 | string | Mandatory fixed portion of UDI |
   | I | 0..1 | uri | UDI Issuing Organization |
   | I | 0..1 | uri | Regional UDI authority |
   | ΣI | 0..1 | base64Binary | UDI Machine Readable Barcode String |
   | ΣI | 0..1 | string | UDI Human Readable Barcode String |
  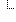 | I | 0..1 | code | barcode | rfid | manual + Binding: UDIEntryType (required) |
  | ?!ΣI | 0..1 | code | active | inactive | entered-in-error | unknown Binding: FHIRDeviceStatus (required) |
  | I | 0..* | CodeableConcept | online | paused | standby | offline | not-ready | transduc-discon | hw-discon | off Binding: FHIRDeviceStatusReason (extensible) |
  | I | 0..1 | string | The distinct identification string |
  | I | 0..1 | string | Name of device manufacturer |
  | I | 0..1 | dateTime | Date when the device was made |
  | I | 0..1 | dateTime | Date and time of expiry of this device (if applicable) |
  | I | 0..1 | string | Lot number of manufacture |
  | I | 0..1 | string | Serial number assigned by the manufacturer |
  | I | 0..* | BackboneElement | The name of the device as given by the manufacturer |
   | 0..1 | string | Unique id for inter-element referencing | |
   | I | 0..* | Extension | Additional content defined by implementations |
   | ?!ΣI | 0..* | Extension | Extensions that cannot be ignored even if unrecognized |
   | I | 1..1 | string | The name of the device |
  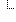 | I | 1..1 | code | udi-label-name | user-friendly-name | patient-reported-name | manufacturer-name | model-name | other Binding: DeviceNameType (required) |
  | I | 0..1 | string | The model number for the device |
  | I | 0..1 | string | The part number of the device |
  | I | 0..1 | CodeableConcept | The kind or type of device Binding: DeviceType (example) |
  | I | 0..* | BackboneElement | The capabilities supported on a device, the standards to which the device conforms for a particular purpose, and used for the communication |
   | 0..1 | string | Unique id for inter-element referencing | |
   | I | 0..* | Extension | Additional content defined by implementations |
   | ?!ΣI | 0..* | Extension | Extensions that cannot be ignored even if unrecognized |
   | I | 1..1 | CodeableConcept | The standard that is used to operate and communicate |
  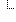 | I | 0..1 | string | The version of the standard that is used to operate and communicate |
  | I | 0..* | BackboneElement | The actual design of the device or software version running on the device |
   | 0..1 | string | Unique id for inter-element referencing | |
   | I | 0..* | Extension | Additional content defined by implementations |
   | ?!ΣI | 0..* | Extension | Extensions that cannot be ignored even if unrecognized |
   | I | 0..1 | CodeableConcept | The type of the device version |
   | I | 0..1 | Identifier | A single component of the device version |
  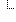 | I | 1..1 | string | The version text |
  | I | 0..* | BackboneElement | The actual configuration settings of a device as it actually operates, e.g., regulation status, time properties |
   | 0..1 | string | Unique id for inter-element referencing | |
   | I | 0..* | Extension | Additional content defined by implementations |
   | ?!ΣI | 0..* | Extension | Extensions that cannot be ignored even if unrecognized |
   | I | 1..1 | CodeableConcept | Code that specifies the property DeviceDefinitionPropetyCode (Extensible) |
   | I | 0..* | Quantity | Property value as a quantity |
   | I | 0..* | CodeableConcept | Property value as a code, e.g., NTP4 (synced to NTP) |
  | I | 0..1 | Reference(Patient) | Patient to whom Device is affixed |
  | I | 0..1 | Reference(Organization) | Organization responsible for device |
  | I | 0..* | ContactPoint | Details for human/organization for support |
  | I | 0..1 | Reference(Location) | Where the device is found |
  | I | 0..1 | uri | Network address to contact device |
  | I | 0..* | Annotation | Device notes and comments |
  | ΣI | 0..* | CodeableConcept | Safety Characteristics of Device |
  | I | 0..1 | Reference(Device) | The parent device |
 Documentation for this format Documentation for this format | ||||
This structure is derived from Device
Differential View
This structure is derived from Device
| Name | Flags | Card. | Type | Description & Constraints |
|---|---|---|---|---|
 | 0..* | Device | Item used in healthcare | |
 Documentation for this format Documentation for this format | ||||
Snapshot View
| Name | Flags | Card. | Type | Description & Constraints |
|---|---|---|---|---|
 | I | 0..* | Device | Item used in healthcare |
  | Σ | 0..1 | string | Logical id of this artifact |
  | ΣI | 0..1 | Meta | Metadata about the resource |
  | ?!ΣI | 0..1 | uri | A set of rules under which this content was created |
  | I | 0..1 | code | Language of the resource content Binding: CommonLanguages (preferred) Max Binding: AllLanguages |
  | I | 0..1 | Narrative | Text summary of the resource, for human interpretation |
  | 0..* | Resource | Contained, inline Resources | |
  | I | 0..* | Extension | Additional content defined by implementations |
  | ?!I | 0..* | Extension | Extensions that cannot be ignored |
  | I | 0..* | Identifier | Instance identifier |
  | I | 0..1 | Reference(DeviceDefinition) | The reference to the definition for the device |
  | ΣI | 0..* | BackboneElement | Unique Device Identifier (UDI) Barcode string |
   | 0..1 | string | Unique id for inter-element referencing | |
   | I | 0..* | Extension | Additional content defined by implementations |
   | ?!ΣI | 0..* | Extension | Extensions that cannot be ignored even if unrecognized |
   | ΣI | 0..1 | string | Mandatory fixed portion of UDI |
   | I | 0..1 | uri | UDI Issuing Organization |
   | I | 0..1 | uri | Regional UDI authority |
   | ΣI | 0..1 | base64Binary | UDI Machine Readable Barcode String |
   | ΣI | 0..1 | string | UDI Human Readable Barcode String |
   | I | 0..1 | code | barcode | rfid | manual + Binding: UDIEntryType (required) |
  | ?!ΣI | 0..1 | code | active | inactive | entered-in-error | unknown Binding: FHIRDeviceStatus (required) |
  | I | 0..* | CodeableConcept | online | paused | standby | offline | not-ready | transduc-discon | hw-discon | off Binding: FHIRDeviceStatusReason (extensible) |
  | I | 0..1 | string | The distinct identification string |
  | I | 0..1 | string | Name of device manufacturer |
  | I | 0..1 | dateTime | Date when the device was made |
  | I | 0..1 | dateTime | Date and time of expiry of this device (if applicable) |
  | I | 0..1 | string | Lot number of manufacture |
  | I | 0..1 | string | Serial number assigned by the manufacturer |
  | I | 0..* | BackboneElement | The name of the device as given by the manufacturer |
   | 0..1 | string | Unique id for inter-element referencing | |
   | I | 0..* | Extension | Additional content defined by implementations |
   | ?!ΣI | 0..* | Extension | Extensions that cannot be ignored even if unrecognized |
   | I | 1..1 | string | The name of the device |
   | I | 1..1 | code | udi-label-name | user-friendly-name | patient-reported-name | manufacturer-name | model-name | other Binding: DeviceNameType (required) |
  | I | 0..1 | string | The model number for the device |
  | I | 0..1 | string | The part number of the device |
  | I | 0..1 | CodeableConcept | The kind or type of device Binding: DeviceType (example) |
  | I | 0..* | BackboneElement | The capabilities supported on a device, the standards to which the device conforms for a particular purpose, and used for the communication |
   | 0..1 | string | Unique id for inter-element referencing | |
   | I | 0..* | Extension | Additional content defined by implementations |
   | ?!ΣI | 0..* | Extension | Extensions that cannot be ignored even if unrecognized |
   | I | 1..1 | CodeableConcept | The standard that is used to operate and communicate |
   | I | 0..1 | string | The version of the standard that is used to operate and communicate |
  | I | 0..* | BackboneElement | The actual design of the device or software version running on the device |
   | 0..1 | string | Unique id for inter-element referencing | |
   | I | 0..* | Extension | Additional content defined by implementations |
   | ?!ΣI | 0..* | Extension | Extensions that cannot be ignored even if unrecognized |
   | I | 0..1 | CodeableConcept | The type of the device version |
   | I | 0..1 | Identifier | A single component of the device version |
   | I | 1..1 | string | The version text |
  | I | 0..* | BackboneElement | The actual configuration settings of a device as it actually operates, e.g., regulation status, time properties |
   | 0..1 | string | Unique id for inter-element referencing | |
   | I | 0..* | Extension | Additional content defined by implementations |
   | ?!ΣI | 0..* | Extension | Extensions that cannot be ignored even if unrecognized |
   | I | 1..1 | CodeableConcept | Code that specifies the property DeviceDefinitionPropetyCode (Extensible) |
   | I | 0..* | Quantity | Property value as a quantity |
   | I | 0..* | CodeableConcept | Property value as a code, e.g., NTP4 (synced to NTP) |
  | I | 0..1 | Reference(Patient) | Patient to whom Device is affixed |
  | I | 0..1 | Reference(Organization) | Organization responsible for device |
  | I | 0..* | ContactPoint | Details for human/organization for support |
  | I | 0..1 | Reference(Location) | Where the device is found |
  | I | 0..1 | uri | Network address to contact device |
  | I | 0..* | Annotation | Device notes and comments |
  | ΣI | 0..* | CodeableConcept | Safety Characteristics of Device |
  | I | 0..1 | Reference(Device) | The parent device |
 Documentation for this format Documentation for this format | ||||
Other representations of profile: Schematron
| Path | Conformance | ValueSet |
| Device.language | preferred | CommonLanguages Max Binding: AllLanguages |
| Device.udiCarrier.entryType | required | UDIEntryType |
| Device.status | required | FHIRDeviceStatus |
| Device.statusReason | extensible | FHIRDeviceStatusReason |
| Device.deviceName.type | required | DeviceNameType |
| Device.type | example | DeviceType |
| Id | Path | Details | Requirements |