This page is part of the International Patient Summary Implementation Guide (v1.1.0: STU 1) based on FHIR R4. This is the current published version. For a full list of available versions, see the Directory of published versions 
| Page standards status: Informative |
The IPS is composed by the following sections described below.
Figure 2: The IPS composition
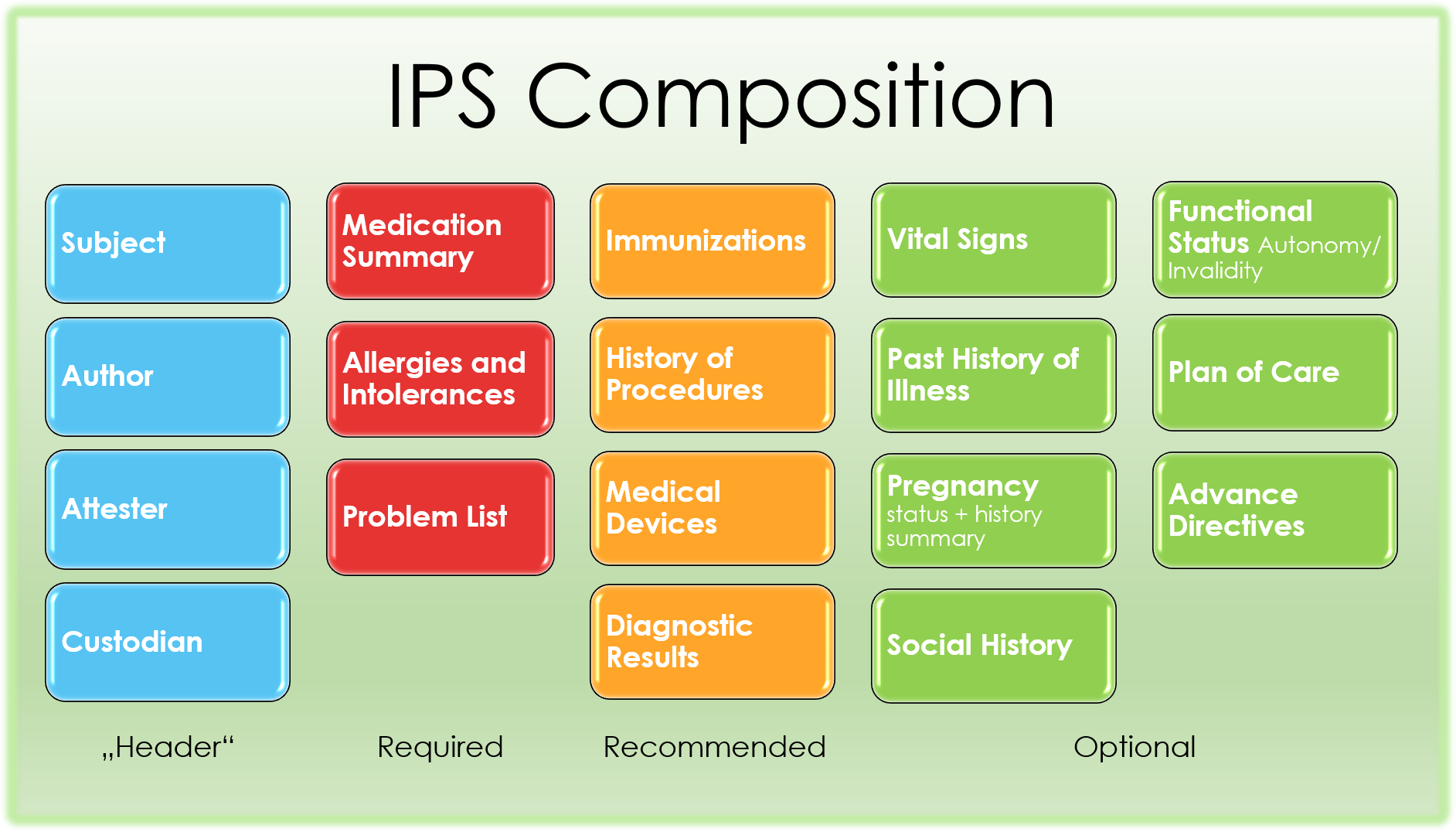
The medication summary section contains a description of the patient’s medications relevant for the scope of the patient summary.
The actual content could depend on the jurisdiction, it could report:
In all those cases however medications are documented in the Patient Summary as medication statements.
The entries of this section must be one of the choices below:
This section documents the relevant allergies or intolerances (conditions) for a patient, describing the kind of reaction (e.g. rash, anaphylaxis,..); preferably the agents that cause it; and optionally the criticality and the certainty of the allergy. At a minimum, it should list currently active and any relevant historical allergies and adverse reactions. If no information about allergies is available, or if no allergies are known this should be clearly documented in the section.
The IPS problem section lists and describes clinical problems or conditions currently being monitored for the patient.
The Immunizations Section defines a patient’s current immunization status and pertinent immunization history. The primary use case for the Immunization Section is to enable communication of a patient’s immunization status. The section includes current immunization status and the entire clinically pertinent immunization history that is known.
The History of Procedures Section contains a description of the patient past procedures that are pertinent to the scope of the IPS.
Procedures may refer for example to:
The medical devices section contains narrative text and coded entries describing the patient history of medical device use.
This section assembles relevant observation results collected on the patient or produced on in-vitro biologic specimens collected from the patient. Some of these results may be laboratory results, others may be anatomic pathology results, and others, radiology results.
This section includes entry choices to carry result observations (using Observation or referenced observations in DiagnosticReport) from:
A generic result entry is also supported.
The Vital signs section includes blood pressure, body temperature, heart rate, and respiratory rate. It may also include other clinical findings, such as height, weight, body mass index, head circumference, and pulse oximetry. In particular, notable vital signs or physical findings such as the most recent, maximum and/or minimum, baseline, or relevant trends may be included
The History of Past Illness section contains a description of the conditions the patient suffered in the past
The pregnancy status and history is comprised of
The social history is as of now comprised of
The plan of care section contains a narrative description of the expectations for care including proposals, goals, and order requests for monitoring, tracking, or improving the condition of the patient.
The functional status section contains a narrative description of capability of the patient to perform acts of daily living, including possible needs of the patient to be continuously assessed by third parties. The invalidity status may in fact influence decisions about how to administer treatments. Profiles to express disabilities and functional assessments will be specified by future versions of this guide.
The advance directives section contains a narrative description of patient’s advance directives.
The profiles that have been defined for this implementation guide are listed here.
Following are the profiles that have been defined for each section. (R) denotes a required section (i.e. must be present in an IPS), (S) denotes a recommended section, the others are optional: