This page is part of the International Patient Summary Implementation Guide (v0.2.0: STU 1 Ballot 2) based on FHIR (HL7® FHIR® Standard) R4. The current version which supersedes this version is 2.0.0. For a full list of available versions, see the Directory of published versions
SD.7 StructureDefinition-device-observer-uv-ips
This profile represents the constraints applied to the Device resource by the IPS project, which specifies an international patient summary based on the FHIR standard R4.;
This profile describes a device that plays the role of observer.
Conformance resource variables defined here
SD.7.1 Formal Views of Profile Content
Description of Profiles, Differentials, and Snapshots.
The official URL for this profile is: http://hl7.org/fhir/uv/ips/StructureDefinition/device-observer-uv-ips
Published on Tue Aug 21 16:07:16 AEST 2018 as a draft by .
This profile builds on Device
Differential View
Snapshot View
| Name | Flags | Card. | Type | Description & Constraints |
|---|---|---|---|---|
 | I | 0..* | Item used in healthcare | |
  | Σ | 0..1 | id | Logical id of this artifact |
  | Σ | 0..1 | Meta | Metadata about the resource |
  | ?!Σ | 0..1 | uri | A set of rules under which this content was created |
  | 0..1 | code | Language of the resource content Binding: Common Languages (preferred) | |
  | 0..1 | Narrative | Text summary of the resource, for human interpretation | |
  | 0..* | Resource | Contained, inline Resources | |
  | 0..* | Extension | Additional Content defined by implementations | |
  | ?! | 0..* | Extension | Extensions that cannot be ignored |
  | S | 0..* | Identifier | Instance identifier |
  | 0..1 | Reference(DeviceDefinition) | The reference to the definition for the device | |
  | ΣI | 0..* | BackboneElement | Unique Device Identifier (UDI) Barcode string |
   | 0..1 | string | xml:id (or equivalent in JSON) | |
   | 0..* | Extension | Additional content defined by implementations | |
   | ?!Σ | 0..* | Extension | Extensions that cannot be ignored if unrecognized |
   | Σ | 0..1 | string | Mandatory fixed portion of UDI |
   | 0..1 | uri | UDI Issuing Organization | |
   | 0..1 | uri | Regional UDI authority | |
   | Σ | 0..1 | base64Binary | UDI Machine Readable Barcode String |
   | Σ | 0..1 | string | UDI Human Readable Barcode String |
  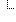 | 0..1 | code | barcode | rfid | manual + Binding: UDIEntryType (required) | |
  | ?!Σ | 0..1 | code | active | inactive | entered-in-error | unknown Binding: FHIRDeviceStatus (required) |
  | 0..* | CodeableConcept | online | paused | standby | offline | not-ready | transduc-discon | hw-discon | off Binding: FHIRDeviceStatusReason (extensible) | |
  | 0..1 | string | The distinct identification code for a biological product regulated as a device | |
  | S | 0..1 | string | Name of device manufacturer |
  | 0..1 | dateTime | Date when the device was made | |
  | 0..1 | dateTime | Date and time of expiry of this device (if applicable) | |
  | 0..1 | string | Lot number of manufacture | |
  | 0..1 | string | Serial number assigned by the manufacturer | |
  | I | 0..* | BackboneElement | The name of the device as given by the manufacturer |
   | 0..1 | string | xml:id (or equivalent in JSON) | |
   | 0..* | Extension | Additional content defined by implementations | |
   | ?!Σ | 0..* | Extension | Extensions that cannot be ignored if unrecognized |
   | 1..1 | string | The name of the device | |
  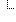 | 1..1 | code | udi-label-name | user-friendly-name | patient-reported-name | manufacturer-name | model-name | other Binding: DeviceNameType (required) | |
  | S | 0..1 | string | The model number for the device |
  | 0..1 | string | The part number of the device | |
  | 0..1 | CodeableConcept | The kind or type of device Binding: FHIRDeviceTypes (example) | |
  | I | 0..* | BackboneElement | The capabilities supported on a device, the standards to which the device conforms for a particular purpose, and used for the communication |
   | 0..1 | string | xml:id (or equivalent in JSON) | |
   | 0..* | Extension | Additional content defined by implementations | |
   | ?!Σ | 0..* | Extension | Extensions that cannot be ignored if unrecognized |
   | 1..1 | CodeableConcept | The standard that is used to operate and communicate | |
  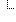 | 0..1 | string | The version of the standard that is used to operate and communicate | |
  | I | 0..* | BackboneElement | The actual design of the device or software version running on the device |
   | 0..1 | string | xml:id (or equivalent in JSON) | |
   | 0..* | Extension | Additional content defined by implementations | |
   | ?!Σ | 0..* | Extension | Extensions that cannot be ignored if unrecognized |
   | 0..1 | CodeableConcept | The type of the device version | |
   | 0..1 | Identifier | A single component of the device version | |
  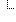 | 1..1 | string | The version text | |
  | I | 0..* | BackboneElement | The actual configuration settings of a device as it actually operates, e.g., regulation status, time properties |
   | 0..1 | string | xml:id (or equivalent in JSON) | |
   | 0..* | Extension | Additional content defined by implementations | |
   | ?!Σ | 0..* | Extension | Extensions that cannot be ignored if unrecognized |
   | 1..1 | CodeableConcept | Code that specifies the property DeviceDefinitionPropetyCode (Extensible) | |
   | 0..* | Quantity | Property value as a quantity | |
   | 0..* | CodeableConcept | Property value as a code, e.g., NTP4 (synced to NTP) | |
  | 0..1 | Reference(Patient) | Patient to whom Device is affixed | |
  | 0..1 | Reference(Organization) | Organization responsible for device | |
  | 0..* | ContactPoint | Details for human/organization for support | |
  | 0..1 | Reference(Location) | Where the device is found | |
  | 0..1 | uri | Network address to contact device | |
  | 0..* | Annotation | Device notes and comments | |
  | Σ | 0..* | CodeableConcept | Safety Characteristics of Device |
  | 0..1 | Reference(Device) | The parent device | |
 Documentation for this format Documentation for this format | ||||
Downloads: StructureDefinition: (XML, JSON), Schema: XML Schematron



