This page is part of the Clinical Guidelines (v1.0.0: STU 1) based on FHIR (HL7® FHIR® Standard) R4. The current version which supersedes this version is 2.0.0. For a full list of available versions, see the Directory of published versions
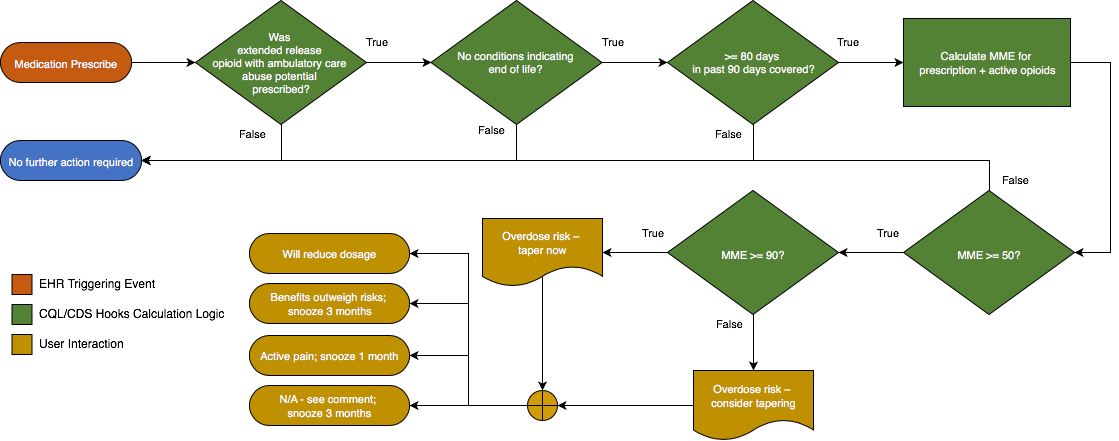
This example illustrates the use of FHIR resources to represent computable content for Recommendation 5 of the CDC Opioid Prescribing Guideline:
CDC Opioid Prescribing Guideline
The examples in this section were funded by The Office of the National Coordinator for Health Information Technology (ONC), contract numbers HHSP233201800320G, HHSP233201700044C, and D15PD00739
When opioids are started, providers should prescribe the lowest effective dosage. Providers should use caution when prescribing opioids at any dosage, should carefully reassess evidence of individual benefits and risks when considering increasing dosage to ≥50 morphine milligram equivalents (MME)/day, and should avoid increasing dosage to ≥90 MME/day or carefully justify a decision to titrate dosage to >90 MME/day
(recommendation category: A, evidence type: 3).
For both recommendations, provide the information used to calculate the MME as a table of results to provide the clinician with sufficient information to understand how the equivalence was calculated, as well as the source of the data used in the calculation.
In addition, provide links to the CDC Guidance, as well as the MME conversion table.
For both recommendations, one of the following responses should be required: