This page is part of the Making EHR Data MOre available for Research and Public Health (MedMorph) (v0.2.0: STU 1 Draft) based on FHIR (HL7® FHIR® Standard) R4. The current version which supersedes this version is 1.0.0. For a full list of available versions, see the Directory of published versions
For Health Care Surveys: Identify and collect hospital (emergency department and inpatient care) and ambulatory care practice data and electronically report them to a system hosted at the federal level. Electronic reporting will increase the response rate of sampled hospitals and ambulatory health care providers to the National Hospital Care Survey (NHCS) and the National Ambulatory Medical Care Survey (NAMCS). This will also increase the volume, quality, completeness, and timeliness of the data submitted to the NHCS and NAMCS. Electronic reporting via automated means (without provider involvement) will reduce the burden associated with survey participation and reduce costs associated with recruiting hospital and ambulatory health care providers.
For Hepatitis C: Leverage existing FHIR infrastructure including electronic case reporting to improve the availability of data that advance national priorities such as eliminating hepatitis C as a public threat in the US, especially if it becomes a chronic condition when not treated acutely. The goal is to optimize access to hepatitis C data already captured within the EHR.
For Cancer Registry Rporting: Leverage existing FHIR infrastructure to transmit cancer case information primarily from ambulatory care practices to state cancer registries. The intent is to automate and provide access to data not currently available to public health and research. This will not replace hospital registry reporting methods which are working well. Automation of cancer case registry reporting will reduce the burden of manual or other non-standardized data collection processes that currently exist.
For Research: Onboard a new research data partner to join a research network, and subsequently allow those researchers within the network the ability to submit specific research questions and receive responses.
By leveraging FHIR-based APIs, research and public health organizations can automate data collection (based on specific events and conditions in clinical workflows) and submission of the collected data, complying with all necessary authorities and policies applicable. A more detailed discussion of the MedMorph Use Cases can be found here.
This section defines the Actors and Systems that interact within the MedMorph Reference Architecture to realize the capabilities of the use cases listed above.
Electronic Health Record (EHR): A system used in care delivery for patients and that captures and stores data about patients and makes the information available instantly and securely to authorized users. While an EHR does contain the medical and treatment histories of patients, an EHR system is built to go beyond standard clinical data collected in a provider’s provision of care location and can be inclusive of a broader view of a patient’s care. EHRs are a vital part of health IT and can:
A FHIR Enabled EHR exposes FHIR APIs providing a mechanism for other systems to interact with the EHR and exchange data. FHIR APIs provide well defined mechanisms to read and write data. The FHIR APIs are protected by an Authorization Server which authenticates and authorizes users or systems prior to accessing the data.
Provider: A person who delivers health care to a patient. In addition to delivering care, the provider also updates the EHR with the patient data captured during the workflow and signs off on the content added, deleted, or modified in the patient record once it is updated. The provider can be the individual doctor, nurse, or a combination of different people that are part of the care team.
Knowledge Artifact Repository: Enables the full spectrum of reporting and querying for programs, studies, and initiatives. A Knowledge Artifact Repository represents the system that is used to distribute metadata such as surveys, trigger codes, timing constraints, decision support artifacts, and other knowledge artifacts to:
Note: Knowledge artifacts do not contain any PHI or PII information
Backend Service App: A system that resides within the clinical care setting and performs the reporting functions to public health and/or research registries. The Backend Service App uses the information supplied by the knowledge artifact repository along with applicable authorities and policies to determine when reporting needs to be done, what data needs to be reported, how the data needs to be reported, and where the data should be reported. The term “Backend Service” is used to refer to the fact that the system does not require user intervention to perform reporting. The term “App” is used to indicate that it is similar to a SMART on FHIR App which can be distributed to clinical care via EHR vendor-specified processes. The EHR vendor-specified processes enable the Backend Service App the ability to use the EHR’s FHIR APIs to access data. The healthcare organization is responsible for choosing/developing and maintaining the Backend Service App within the organization.
Trust Service Provider: Trust Service Provider affords capabilities that can be used to pseudonymize, anonymize, de-identify, hash, or re-link data that is submitted to public health and/or research organizations. These capabilities are called Trust Services. Trust Services are used, when appropriate, by the Backend Service App.
Trusted Third Party: A system (e.g., Health Information Exchange (HIE), Reportable Condition Knowledge Management System (RCKMS)/Association of Public Health Laboratories (APHL) Informatics Messaging Services (AIMS) Platform) at an intermediary organization that serves as a conduit to exchange data between healthcare organizations and an endpoint. Trusted Third Parties perform the intermediary functions (e.g., apply business logic and inform the Reportability Response) using appropriate authorities and policies.
Data Repository: A system receiving the data from the clinical care system. A Data Repository is used to represent systems such as cancer registries, National Healthcare Survey data stores, surveillance systems, and electronic vital records systems. Data Repositories are actively managed and are used to receive data, store data, and perform analysis as appropriate. These data repositories could be operated or accessed by Public Health Authority (or their designated organizations) or research organizations, with appropriate authorities and policies.
Public Health Authority (PHA): A government or a government-designated organization allowed to receive the data from Trusted Third Parties or provider organizations using appropriate authorities and policies. PHA may also analyze the data and initiate responses back to clinical care. For more detailed information on PHA please refer to https://www.hhs.gov/hipaa/for-professionals/special-topics/public-health/index.html.
Research Organization: An organization that can access the data from clinical care or data repositories for research purposes with appropriate data use agreements, authorities, and policies.
This section outlines the high level interactions between the various MedMorph Actors and Systems defined above. These interactions are shown in the Figure 3.0 below along with the descriptions for each step.
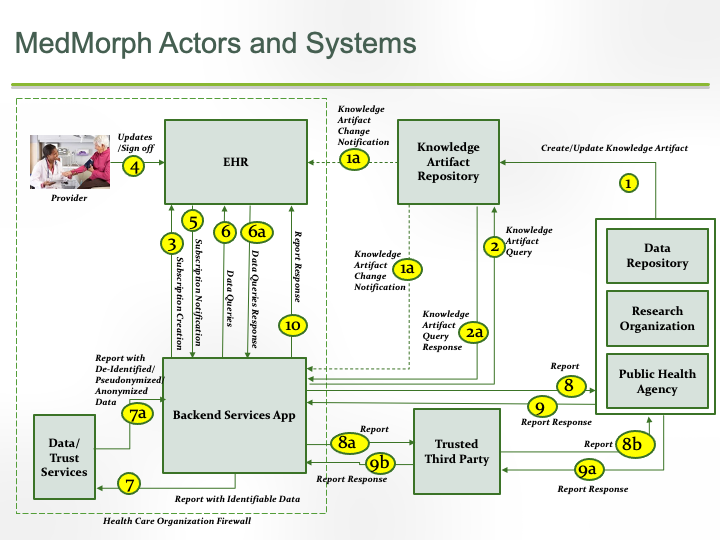
The descriptions for each step in the above diagram are described below:
The next few sections considers subsets of the above the interactions for ease of understanding and documenting detailed interactions using applicable FHIR mechanisms.
The following section outlines each of the MedMorph workflows, actors, and their interactions based on MedMorph Use Cases.
The Provisioning Workflow involves the activities whereby a PHA or a Research Organization publishes a Knowledge Artifact(s) specific to a use case. The published Knowledge Artifact(s) is then downloaded by the Backend Service App. The Backend Service App may subscribe to notifications from the EHR based on data present within the Knowledge Artifact. The actors and steps involved in the workflow are shown in Figure 3.1 below.
The Notification Workflow involves the activities whereby a provider, as part of the care delivery process, updates a patient’s medical record within an EHR. The EHR, based on the changes to the medical record and existing subscriptions, will notify the Backend Service App of the changes in the medical record. The Backend Service App will perform actions such as querying the EHR for additional data, scheduling a reporting job, and creating a report for submission based on the notification and the Knowledge Artifact data. The actors and steps involved in the workflow are shown in Figure 3.2 below.
The Report Creation Workflow involves the activities whereby the Backend Service App collects the data necessary for creating the report to be submitted to the PHA or Research Organization. The Backend Service App may then perform additional activities such as pseudonymization, anonymization or de-identification based on the specific use case and knowledge artifact. Finally, the Backend Service App assembles the data in the required reporting format for submission. The actors and steps involved in the workflow are shown in Figure 3.3 below.
The Data Submission Workflow involves the activities whereby the Backend Service App packages the data and submits the data to the PHA or Research Organization. The data submission may occur through a Trusted Third Party. The actors and steps involved in the workflow are shown in Figure 3.4 below.
The Receiving Response/Acknowledgement Workflow involves the activities whereby the PHA or Research Organization provides a response back to clinical care. This workflow depicts the PUSH method (i.e., PUSH from PHA/Research Organization to Clinical Care). The actors and steps involved in the workflow are shown in Figure 3.5 below.
The Receiving Response/Acknowledgement Workflow involves the activities whereby the PHA or Research Organization provides a response back to clinical care. This workflow depicts the PULL method (i.e., Clinical Care PULLS the response from PHA/Research Organization). The actors and steps involved in the workflow are shown in Figure 3.6 below.