This page is part of the Profiles for ICSR Transfusion and Vaccination Adverse Event Detection and Reporting (v0.1.0: STU 1 Ballot 1) based on FHIR R4. The current version which supercedes this version is 1.0.0. For a full list of available versions, see the Directory of published versions 
This FHIR Implementation Guide details a process where adverse event (AE) individual case safety reports (ICSR) are generated from EHR data. There are two main components to this process: detection and reporting. To support AE detection, this IG includes a set of Clinical Quality Language (CQL) algorithms which can be run on FHIR data. To support AE reporting, this IG includes a set of profiles that enables the creation of a FHIR-based ICSR report and mappings to ICH ICSR specification (FAERS and VAERS implementation of those specifications). This work was developed as part of the FDA’s Center for Biologic Evaluation and Research (CBER) Biologics Effectiveness and Safety (BEST) initiatve. This IG currently focuses on post-vaccination and post-tranfusion AE reporting.
Adverse events due to biological products are underreported to regulatory authorities and the data reported may not always contain adequate information for decision-making. Use of EHR FHIR data could improve reporting of adverse events and minimize the burden of identification on practitioners.
This IG depends on:
We consider the AE Detection and Submission workflow to be a type of MedMorph’s Data Submission Workflow.
NOTE: The diagram below is a UML activity diagram. The «use» stereotypes indicate data that is used by the associated activity. If the arrow is pointing to the activity, then the activity is reading that data. If the arrow is pointing away from the activity, then the activity is generating that data.
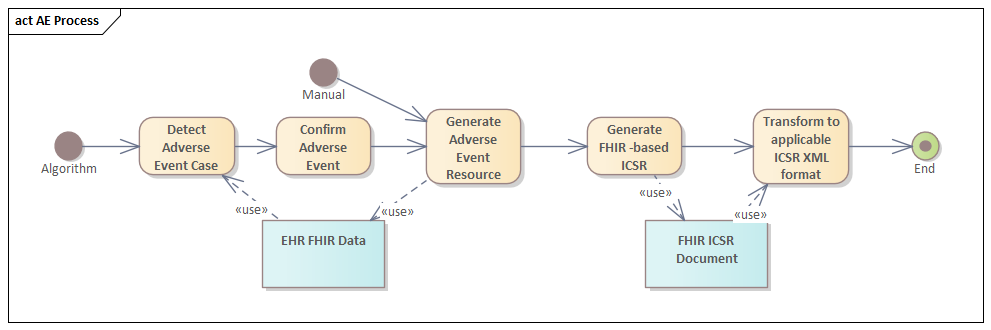
A system can detect AE cases automatically using the FHIR-based CQL algorithms referenced in this IG.
Once an Adverse Event has been detected, a practitioner can review the relevant data and confirm that it is an actual Adverse Event that should be reported.
Once an Adverse Event has been confirmed or if a practitioner manually determines there is an Adverse Event to report, a system can generate an instance of the FHIR AdverseEvent resource.
A system can generate a FHIR-based ICSR document using the profiles referenced in this IG.
A system can further transform the ICSR document to the appropriate XML formal for submission to the FDA FAERS and VAERS systems. This IG provides sample ICSR Mapping XSL transformations.
The development of this Implementation Guide was funded by FDA CBER BEST initiative.