This page is part of the At-Home In-Vitro Test Report (v1.0.0: STU 1) based on FHIR (HL7® FHIR® Standard) R4. The current version which supersedes this version is 1.1.0. For a full list of available versions, see the Directory of published versions
A generic or typical At-Home In-Vitro use case starts when a patient either purchases an over the counter (OTC) At-Home In-Vitro test or receives an ordered At-Home In-Vitro test. The patient interacts with an At-Home In-Vitro Test app. The test result is then either:
A single patient may run more than one test. Each test result is a new interaction with the app.
The patient may be asked questions by the app, such as presence or absence of use case-specific symptoms. The answers are recorded in the app. The questions and answers are considered to be supporting information and similar to “Ask at Order Entry” (AOE) questions. These questions are not considered the same as “Ask at Order Entry” (AOE) questions by lab standards experts because:
This solution of representing the information with the supportingInfo FHIR Extension was chosen during the pandemic as it is a supported way for laboratories to add information to their LIS and subsequently send it out. There was much discussion and long-term this information should be coming from other systems to PH, so the lab does not need to store information just for the sake of forwarding it. For this At-Home Test use case that might be an option, so for this use case it may remain the solution (or it may need to be revisited).
Whether the At-Home In-Vitro Test is ordered or OTC, the app will not have access to the order details. Therefore, the ServiceRequest for the order will not be constrained in this IG.
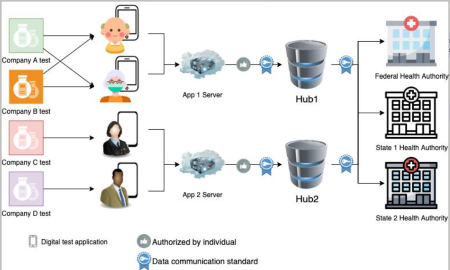
Rather than apps establishing individual relationships with federal and state public health agencies (PHAs), apps negotiate relationships with a data hub or hubs, which then relay the At-Home In-Vitro Test result to federal and state PHAs with whom the hubs have previously established relationships. This reduces the burden on the app manufacturer. App manufacturers may choose to partner with a hub maintained by a third-party to simplify internal operations.
The COVID-19 At-Home In-Vitro use case starts when a patient either purchases an OTC COVID-19 At-Home In-Vitro test or receives an ordered COVID-19 At-Home In-Vitro test. The patient interacts with a COVID-19 At-Home In-Vitro Test app. The test result is then either:
A single patient may run more than one test. Each test result is a new interaction with the app.
The patient is asked questions by the app, such as presence or absence of COVID-19-like symptoms. The answers are recorded in the app.
The apps negotiate relationships with a data hub or hubs, which then relay the At-Home In-Vitro Test result to federal and state PHAs with whom the hubs have previously established relationships.
 |