This page is part of the US Core (v3.0.0: STU3) based on FHIR (HL7® FHIR® Standard) R4. The current version which supersedes this version is 8.0.1. For a full list of available versions, see the Directory of published versions. Page versions: R7 R6 R5 R4 R3
Future of US Core
This section outlines the approach to adding new content to US Core.
Contents
Future of US Core
The US Core FHIR profiles are designed to be the base set of requirements for FHIR implementation in the US. All US Realm implementation guides SHALL use the US Core profiles or SHALL explicitly state why they are unable to use. Throughout the development of US Core, implementers, government, and clinical community have brought forward additional requirements for US Core. This section outlines the approach to growth, and is holding place for items that with additional profiling and testing will be added to US Core.
Growth Path of US Core
The US Core implementation Guide will grow following these steps:
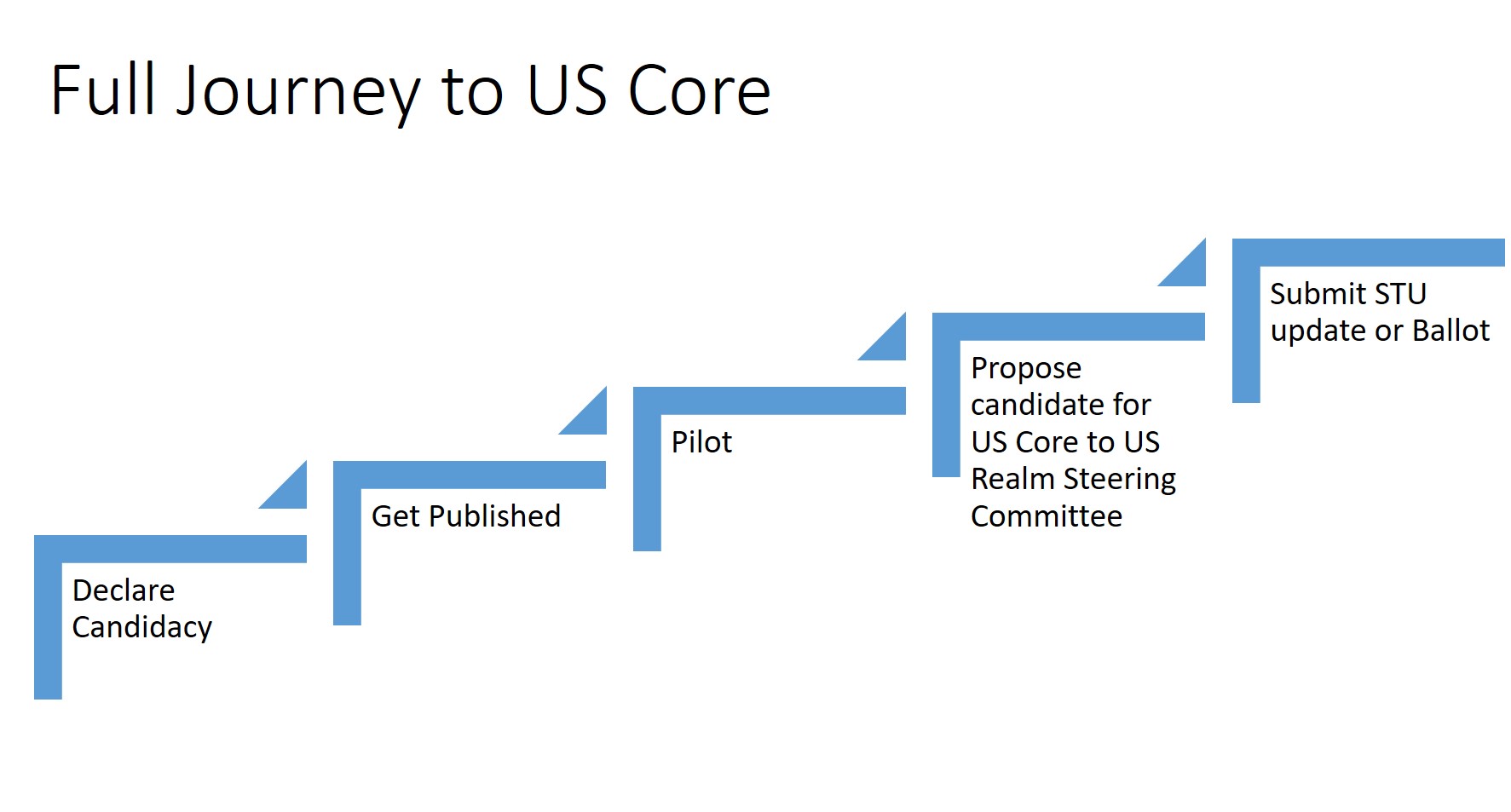
- Declare candidacy - this step can be completed by presenting to the US Realm Steering Committee through a Project Scope Statement.
- Get published - development a formal profile, implementation guide, or get requirements directly published in FHIR Core. The initial publication could be an outside consortium, or vendor publication.
- Pilot - coordinate with 3 or more implementers an in-person or virtual connectathon. This is the time to identify issues with the new proposal.
- Propose candidate for US Core to US Realm Steering Committee - receive formal approval from the US Realm SC to add.
- Submit formal STU comment, or propose through a ballot
A new US Regulatory requirement may jump over some of these steps, however, regulators should be discouraged from skipping pilot testing. Without pilot testing it’s difficult to understand how a change will affect real-world implementation.
In the January ballot of 2019 we tested this process with the FDA requesting US Core include all the component parts of UDI. In prior efforts, the FDA had successfully enhanced the base FHIR specification to include the UDI components, reaching step 3. In the summer of 2019 FDA plans to pilot these proposed new requirements within the Argonaut community the results of which will inform updates to the planned fall 2019 STU release.
Future Requirements Under Considerations
Pilot Testing
Additional UDI elements in a US Core Device Profile
Candidates under consideration
The following items were submitted during a US Core ballot or STU comment. Additional requirements gathering is required before testing may occur on these items:
- ServiceRequest - The CDS hooks community, and other implementers are gathering requirements for the ServiceRequest Resource.



