This page is part of the Breast Cancer Data Logical Models and FHIR Profiles (v0.2.0: STU 1 Draft) based on FHIR (HL7® FHIR® Standard) R3. No current official version has been published yet. For a full list of available versions, see the Directory of published versions
The official URL for this logical model is:
http://hl7.org/fhir/us/breastcancer/StructureDefinition/cimi-entity-MedicationIngredient-model
Specifies an material component in a medication.
This logical model was published on Fri Aug 17 00:00:00 AEST 2018 as a draft by The HL7 Cancer Interoperability Group sponsored by Clinical Interoperability Council Work Group (CIC).
This IG does not define a corresponding profile for this logical model.
| Name | Flags | Card. | Type | Description & Constraints |
|---|---|---|---|---|
 | 0..* | |||
 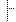 | 1..1 | Coding, Reference(BC Substance Logical Model | BC Medication Logical Model) | Choice of types representing specifies an material component in a medication. | |
 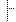 | 1..1 | http://hl7.org/fhir/us/breastcancer/StructureDefinition/shr-core-Ratio-model | The amount of an ingredient in a mixture, as a ratio. For example, 250 mg per tablet is expressed as a ratio where the numerator is 250mg and the denominator is 1 tablet. | |
 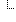 | 1..1 | boolean | True if the ingredient is an active ingredient in the medication. | |
 Documentation for this format Documentation for this format | ||||
Downloads: StructureDefinition: (XML, JSON, TTL)