This page is part of the FHIR Specification (v3.0.2: STU 3). The current version which supercedes this version is 5.0.0. For a full list of available versions, see the Directory of published versions  . Page versions: R5 R4B R4 R3
. Page versions: R5 R4B R4 R3
Work Group Orders and Observations  & Clinical Genomics & Clinical Genomics  | Ballot Status: Informative |
The Diagnostics Module provides an overview and guide to the FHIR content that addresses ordering and reporting of clinical diagnostics including laboratory testing, imaging and genomics.
The Diagnostics module covers the following resources:
The diagnostic resources and their relationships are shown below. The arrows represent the direction of the references between resources (for example, DiagnosticReport references ProcedureRequest). See the Workflow Module for information about the coordination of activities such as ordering and fulfilling of diagnostics.
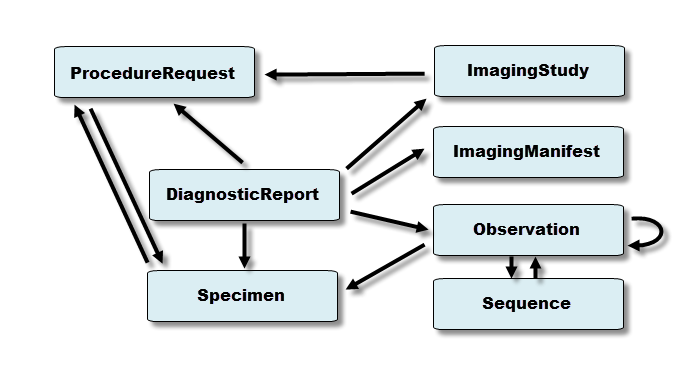
Note: See the Genomics Implementation Guidance for additional information about how to use the Diagnostic resources for Clinical Genomic Reporting and Analysis.
| Name | Aliases | Description |
| Observation | Vital Signs, Measurement, Results | Measurements and simple assertions made about a patient, device or other subject. |
| DiagnosticReport | Report, Test, Result, Results, Labs | The findings and interpretation of diagnostic tests performed on patients, groups of patients, devices, and locations, and/or specimens derived from these. The report includes clinical context such as requesting and provider information, and some mix of atomic results, images, textual and coded interpretations, and formatted representation of diagnostic reports. |
| ProcedureRequest | diagnostic request | A record of a request for diagnostic investigations, treatments, or operations to be performed. |
| ImagingStudy | Representation of the content produced in a DICOM imaging study. A study comprises a set of series, each of which includes a set of Service-Object Pair Instances (SOP Instances - images or other data) acquired or produced in a common context. A series is of only one modality (e.g. X-ray, CT, MR, ultrasound), but a study may have multiple series of different modalities. | |
| ImagingManifest | Manifest, XDS-I summary, Key Images | A text description of the DICOM SOP instances selected in the ImagingManifest; or the reason for, or significance of, the selection. |
| Sequence | Raw data describing a biological sequence. | |
| Specimen | A sample to be used for analysis. | |
| BodySite | anatomical location | Record details about the anatomical location of a specimen or body part. This resource may be used when a coded concept does not provide the necessary detail needed for the use case. |
The diagnostic resources often represent patient-specific data, and as such are susceptible to data breaching. Necessary privacy and security provisions must be in place when searching and fetching this information. For more general considerations, see the Security and Privacy module.
Diagnostic resources are commonly used to plan, recommend, order and report clinical diagnostics:
There are many ways to use these resources independently as well. The Observation resource in particular is central to capturing many measurements and events in healthcare and is often used outside the context of diagnostic orders and reports.
The resources that represent the basic information about a patient and a clinical encounter can be found in the Administration Module. Other resources that represent core clinical information generated by healthcare providers during the course of a patient encounter are detailed in the Clinical Summary Module and the Medications Module.