This page is part of the FHIR Specification (v5.0.0-draft-final: Final QA Preview for R5 - see ballot notes). The current version which supercedes this version is 5.0.0. For a full list of available versions, see the Directory of published versions  . Page versions: R5 R4
. Page versions: R5 R4
Biomedical Research and Regulation  Work Group Work Group | Maturity Level: 0 | Trial Use | Security Category: Anonymous | Compartments: Not linked to any defined compartments |
A SubstanceProtein is defined as a single unit of a linear amino acid sequence, or a combination of subunits that are either covalently linked or have a defined invariant stoichiometric relationship. This includes all synthetic, recombinant and purified SubstanceProteins of defined sequence, whether the use is therapeutic or prophylactic. This set of elements will be used to describe albumins, coagulation factors, cytokines, growth factors, peptide/SubstanceProtein hormones, enzymes, toxins, toxoids, recombinant vaccines, and immunomodulators.
For an overview of this resource and others in the Medication Definition domain, also see the module page
Structure
| Name | Flags | Card. | Type | Description & Constraints |
|---|---|---|---|---|
 | TU | DomainResource | A SubstanceProtein is defined as a single unit of a linear amino acid sequence, or a combination of subunits that are either covalently linked or have a defined invariant stoichiometric relationship. This includes all synthetic, recombinant and purified SubstanceProteins of defined sequence, whether the use is therapeutic or prophylactic. This set of elements will be used to describe albumins, coagulation factors, cytokines, growth factors, peptide/SubstanceProtein hormones, enzymes, toxins, toxoids, recombinant vaccines, and immunomodulators Elements defined in Ancestors: id, meta, implicitRules, language, text, contained, extension, modifierExtension | |
 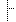 | Σ | 0..1 | CodeableConcept | The SubstanceProtein descriptive elements will only be used when a complete or partial amino acid sequence is available or derivable from a nucleic acid sequence |
 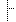 | Σ | 0..1 | integer | Number of linear sequences of amino acids linked through peptide bonds. The number of subunits constituting the SubstanceProtein shall be described. It is possible that the number of subunits can be variable |
 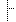 | Σ | 0..* | string | The disulphide bond between two cysteine residues either on the same subunit or on two different subunits shall be described. The position of the disulfide bonds in the SubstanceProtein shall be listed in increasing order of subunit number and position within subunit followed by the abbreviation of the amino acids involved. The disulfide linkage positions shall actually contain the amino acid Cysteine at the respective positions |
  | Σ | 0..* | BackboneElement | This subclause refers to the description of each subunit constituting the SubstanceProtein. A subunit is a linear sequence of amino acids linked through peptide bonds. The Subunit information shall be provided when the finished SubstanceProtein is a complex of multiple sequences; subunits are not used to delineate domains within a single sequence. Subunits are listed in order of decreasing length; sequences of the same length will be ordered by decreasing molecular weight; subunits that have identical sequences will be repeated multiple times |
  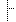 | Σ | 0..1 | integer | Index of primary sequences of amino acids linked through peptide bonds in order of decreasing length. Sequences of the same length will be ordered by molecular weight. Subunits that have identical sequences will be repeated and have sequential subscripts |
   | Σ | 0..1 | string | The sequence information shall be provided enumerating the amino acids from N- to C-terminal end using standard single-letter amino acid codes. Uppercase shall be used for L-amino acids and lowercase for D-amino acids. Transcribed SubstanceProteins will always be described using the translated sequence; for synthetic peptide containing amino acids that are not represented with a single letter code an X should be used within the sequence. The modified amino acids will be distinguished by their position in the sequence |
   | Σ | 0..1 | integer | Length of linear sequences of amino acids contained in the subunit |
   | Σ | 0..1 | Attachment | The sequence information shall be provided enumerating the amino acids from N- to C-terminal end using standard single-letter amino acid codes. Uppercase shall be used for L-amino acids and lowercase for D-amino acids. Transcribed SubstanceProteins will always be described using the translated sequence; for synthetic peptide containing amino acids that are not represented with a single letter code an X should be used within the sequence. The modified amino acids will be distinguished by their position in the sequence |
   | Σ | 0..1 | Identifier | Unique identifier for molecular fragment modification based on the ISO 11238 Substance ID |
   | Σ | 0..1 | string | The name of the fragment modified at the N-terminal of the SubstanceProtein shall be specified |
   | Σ | 0..1 | Identifier | Unique identifier for molecular fragment modification based on the ISO 11238 Substance ID |
   | Σ | 0..1 | string | The modification at the C-terminal shall be specified |
 Documentation for this format Documentation for this format  | ||||
See the Extensions for this resource
UML Diagram (Legend)
XML Template
<SubstanceProtein xmlns="http://hl7.org/fhir"><!-- from Resource: id, meta, implicitRules, and language --> <!-- from DomainResource: text, contained, extension, and modifierExtension --> <sequenceType><!-- 0..1 CodeableConcept The SubstanceProtein descriptive elements will only be used when a complete or partial amino acid sequence is available or derivable from a nucleic acid sequence --></sequenceType> <numberOfSubunits value="[integer]"/><!-- 0..1 Number of linear sequences of amino acids linked through peptide bonds. The number of subunits constituting the SubstanceProtein shall be described. It is possible that the number of subunits can be variable --> <disulfideLinkage value="[string]"/><!-- 0..* The disulphide bond between two cysteine residues either on the same subunit or on two different subunits shall be described. The position of the disulfide bonds in the SubstanceProtein shall be listed in increasing order of subunit number and position within subunit followed by the abbreviation of the amino acids involved. The disulfide linkage positions shall actually contain the amino acid Cysteine at the respective positions --> <subunit> <!-- 0..* This subclause refers to the description of each subunit constituting the SubstanceProtein. A subunit is a linear sequence of amino acids linked through peptide bonds. The Subunit information shall be provided when the finished SubstanceProtein is a complex of multiple sequences; subunits are not used to delineate domains within a single sequence. Subunits are listed in order of decreasing length; sequences of the same length will be ordered by decreasing molecular weight; subunits that have identical sequences will be repeated multiple times --> <subunit value="[integer]"/><!-- 0..1 Index of primary sequences of amino acids linked through peptide bonds in order of decreasing length. Sequences of the same length will be ordered by molecular weight. Subunits that have identical sequences will be repeated and have sequential subscripts --> <sequence value="[string]"/><!-- 0..1 The sequence information shall be provided enumerating the amino acids from N- to C-terminal end using standard single-letter amino acid codes. Uppercase shall be used for L-amino acids and lowercase for D-amino acids. Transcribed SubstanceProteins will always be described using the translated sequence; for synthetic peptide containing amino acids that are not represented with a single letter code an X should be used within the sequence. The modified amino acids will be distinguished by their position in the sequence --> <length value="[integer]"/><!-- 0..1 Length of linear sequences of amino acids contained in the subunit --> <sequenceAttachment><!-- 0..1 Attachment The sequence information shall be provided enumerating the amino acids from N- to C-terminal end using standard single-letter amino acid codes. Uppercase shall be used for L-amino acids and lowercase for D-amino acids. Transcribed SubstanceProteins will always be described using the translated sequence; for synthetic peptide containing amino acids that are not represented with a single letter code an X should be used within the sequence. The modified amino acids will be distinguished by their position in the sequence --></sequenceAttachment> <nTerminalModificationId><!-- 0..1 Identifier Unique identifier for molecular fragment modification based on the ISO 11238 Substance ID --></nTerminalModificationId> <nTerminalModification value="[string]"/><!-- 0..1 The name of the fragment modified at the N-terminal of the SubstanceProtein shall be specified --> <cTerminalModificationId><!-- 0..1 Identifier Unique identifier for molecular fragment modification based on the ISO 11238 Substance ID --></cTerminalModificationId> <cTerminalModification value="[string]"/><!-- 0..1 The modification at the C-terminal shall be specified --> </subunit> </SubstanceProtein>
JSON Template
{ "resourceType" : "SubstanceProtein",
// from Resource: id, meta, implicitRules, and language
// from DomainResource: text, contained, extension, and modifierExtension
"sequenceType" : { CodeableConcept }, // The SubstanceProtein descriptive elements will only be used when a complete or partial amino acid sequence is available or derivable from a nucleic acid sequence
"numberOfSubunits" : <integer>, // Number of linear sequences of amino acids linked through peptide bonds. The number of subunits constituting the SubstanceProtein shall be described. It is possible that the number of subunits can be variable
"disulfideLinkage" : ["<string>"], // The disulphide bond between two cysteine residues either on the same subunit or on two different subunits shall be described. The position of the disulfide bonds in the SubstanceProtein shall be listed in increasing order of subunit number and position within subunit followed by the abbreviation of the amino acids involved. The disulfide linkage positions shall actually contain the amino acid Cysteine at the respective positions
"subunit" : [{ // This subclause refers to the description of each subunit constituting the SubstanceProtein. A subunit is a linear sequence of amino acids linked through peptide bonds. The Subunit information shall be provided when the finished SubstanceProtein is a complex of multiple sequences; subunits are not used to delineate domains within a single sequence. Subunits are listed in order of decreasing length; sequences of the same length will be ordered by decreasing molecular weight; subunits that have identical sequences will be repeated multiple times
"subunit" : <integer>, // Index of primary sequences of amino acids linked through peptide bonds in order of decreasing length. Sequences of the same length will be ordered by molecular weight. Subunits that have identical sequences will be repeated and have sequential subscripts
"sequence" : "<string>", // The sequence information shall be provided enumerating the amino acids from N- to C-terminal end using standard single-letter amino acid codes. Uppercase shall be used for L-amino acids and lowercase for D-amino acids. Transcribed SubstanceProteins will always be described using the translated sequence; for synthetic peptide containing amino acids that are not represented with a single letter code an X should be used within the sequence. The modified amino acids will be distinguished by their position in the sequence
"length" : <integer>, // Length of linear sequences of amino acids contained in the subunit
"sequenceAttachment" : { Attachment }, // The sequence information shall be provided enumerating the amino acids from N- to C-terminal end using standard single-letter amino acid codes. Uppercase shall be used for L-amino acids and lowercase for D-amino acids. Transcribed SubstanceProteins will always be described using the translated sequence; for synthetic peptide containing amino acids that are not represented with a single letter code an X should be used within the sequence. The modified amino acids will be distinguished by their position in the sequence
"nTerminalModificationId" : { Identifier }, // Unique identifier for molecular fragment modification based on the ISO 11238 Substance ID
"nTerminalModification" : "<string>", // The name of the fragment modified at the N-terminal of the SubstanceProtein shall be specified
"cTerminalModificationId" : { Identifier }, // Unique identifier for molecular fragment modification based on the ISO 11238 Substance ID
"cTerminalModification" : "<string>" // The modification at the C-terminal shall be specified
}]
}
"resourceType" : "SubstanceProtein",
// from Resource: id, meta, implicitRules, and language
// from DomainResource: text, contained, extension, and modifierExtension
"sequenceType" : { CodeableConcept }, // The SubstanceProtein descriptive elements will only be used when a complete or partial amino acid sequence is available or derivable from a nucleic acid sequence
"numberOfSubunits" : <integer>, // Number of linear sequences of amino acids linked through peptide bonds. The number of subunits constituting the SubstanceProtein shall be described. It is possible that the number of subunits can be variable
"disulfideLinkage" : ["<string>"], // The disulphide bond between two cysteine residues either on the same subunit or on two different subunits shall be described. The position of the disulfide bonds in the SubstanceProtein shall be listed in increasing order of subunit number and position within subunit followed by the abbreviation of the amino acids involved. The disulfide linkage positions shall actually contain the amino acid Cysteine at the respective positions
"subunit" : [{ // This subclause refers to the description of each subunit constituting the SubstanceProtein. A subunit is a linear sequence of amino acids linked through peptide bonds. The Subunit information shall be provided when the finished SubstanceProtein is a complex of multiple sequences; subunits are not used to delineate domains within a single sequence. Subunits are listed in order of decreasing length; sequences of the same length will be ordered by decreasing molecular weight; subunits that have identical sequences will be repeated multiple times
"subunit" : <integer>, // Index of primary sequences of amino acids linked through peptide bonds in order of decreasing length. Sequences of the same length will be ordered by molecular weight. Subunits that have identical sequences will be repeated and have sequential subscripts
"sequence" : "<string>", // The sequence information shall be provided enumerating the amino acids from N- to C-terminal end using standard single-letter amino acid codes. Uppercase shall be used for L-amino acids and lowercase for D-amino acids. Transcribed SubstanceProteins will always be described using the translated sequence; for synthetic peptide containing amino acids that are not represented with a single letter code an X should be used within the sequence. The modified amino acids will be distinguished by their position in the sequence
"length" : <integer>, // Length of linear sequences of amino acids contained in the subunit
"sequenceAttachment" : { Attachment }, // The sequence information shall be provided enumerating the amino acids from N- to C-terminal end using standard single-letter amino acid codes. Uppercase shall be used for L-amino acids and lowercase for D-amino acids. Transcribed SubstanceProteins will always be described using the translated sequence; for synthetic peptide containing amino acids that are not represented with a single letter code an X should be used within the sequence. The modified amino acids will be distinguished by their position in the sequence
"nTerminalModificationId" : { Identifier }, // Unique identifier for molecular fragment modification based on the ISO 11238 Substance ID
"nTerminalModification" : "<string>", // The name of the fragment modified at the N-terminal of the SubstanceProtein shall be specified
"cTerminalModificationId" : { Identifier }, // Unique identifier for molecular fragment modification based on the ISO 11238 Substance ID
"cTerminalModification" : "<string>" // The modification at the C-terminal shall be specified
}]
}
Turtle Template
@prefix fhir: <http://hl7.org/fhir/> .[ a fhir:SubstanceProtein; fhir:nodeRole fhir:treeRoot; # if this is the parser root # from Resource: .id, .meta, .implicitRules, and .language # from DomainResource: .text, .contained, .extension, and .modifierExtension fhir:sequenceType [ CodeableConcept ] ; # 0..1 The SubstanceProtein descriptive elements will only be used when a complete or partial amino acid sequence is available or derivable from a nucleic acid sequence fhir:numberOfSubunits [ integer ] ; # 0..1 Number of linear sequences of amino acids linked through peptide bonds. The number of subunits constituting the SubstanceProtein shall be described. It is possible that the number of subunits can be variable fhir:disulfideLinkage ( [ string ] ... ) ; # 0..* The disulphide bond between two cysteine residues either on the same subunit or on two different subunits shall be described. The position of the disulfide bonds in the SubstanceProtein shall be listed in increasing order of subunit number and position within subunit followed by the abbreviation of the amino acids involved. The disulfide linkage positions shall actually contain the amino acid Cysteine at the respective positions fhir:subunit ( [ # 0..* This subclause refers to the description of each subunit constituting the SubstanceProtein. A subunit is a linear sequence of amino acids linked through peptide bonds. The Subunit information shall be provided when the finished SubstanceProtein is a complex of multiple sequences; subunits are not used to delineate domains within a single sequence. Subunits are listed in order of decreasing length; sequences of the same length will be ordered by decreasing molecular weight; subunits that have identical sequences will be repeated multiple times fhir:subunit [ integer ] ; # 0..1 Index of primary sequences of amino acids linked through peptide bonds in order of decreasing length. Sequences of the same length will be ordered by molecular weight. Subunits that have identical sequences will be repeated and have sequential subscripts fhir:sequence [ string ] ; # 0..1 The sequence information shall be provided enumerating the amino acids from N- to C-terminal end using standard single-letter amino acid codes. Uppercase shall be used for L-amino acids and lowercase for D-amino acids. Transcribed SubstanceProteins will always be described using the translated sequence; for synthetic peptide containing amino acids that are not represented with a single letter code an X should be used within the sequence. The modified amino acids will be distinguished by their position in the sequence fhir:length [ integer ] ; # 0..1 Length of linear sequences of amino acids contained in the subunit fhir:sequenceAttachment [ Attachment ] ; # 0..1 The sequence information shall be provided enumerating the amino acids from N- to C-terminal end using standard single-letter amino acid codes. Uppercase shall be used for L-amino acids and lowercase for D-amino acids. Transcribed SubstanceProteins will always be described using the translated sequence; for synthetic peptide containing amino acids that are not represented with a single letter code an X should be used within the sequence. The modified amino acids will be distinguished by their position in the sequence fhir:nTerminalModificationId [ Identifier ] ; # 0..1 Unique identifier for molecular fragment modification based on the ISO 11238 Substance ID fhir:nTerminalModification [ string ] ; # 0..1 The name of the fragment modified at the N-terminal of the SubstanceProtein shall be specified fhir:cTerminalModificationId [ Identifier ] ; # 0..1 Unique identifier for molecular fragment modification based on the ISO 11238 Substance ID fhir:cTerminalModification [ string ] ; # 0..1 The modification at the C-terminal shall be specified ] ... ) ; ]
Changes since R4
| SubstanceProtein |
|
See the Full Difference for further information
Structure
| Name | Flags | Card. | Type | Description & Constraints |
|---|---|---|---|---|
 | TU | DomainResource | A SubstanceProtein is defined as a single unit of a linear amino acid sequence, or a combination of subunits that are either covalently linked or have a defined invariant stoichiometric relationship. This includes all synthetic, recombinant and purified SubstanceProteins of defined sequence, whether the use is therapeutic or prophylactic. This set of elements will be used to describe albumins, coagulation factors, cytokines, growth factors, peptide/SubstanceProtein hormones, enzymes, toxins, toxoids, recombinant vaccines, and immunomodulators Elements defined in Ancestors: id, meta, implicitRules, language, text, contained, extension, modifierExtension | |
  | Σ | 0..1 | CodeableConcept | The SubstanceProtein descriptive elements will only be used when a complete or partial amino acid sequence is available or derivable from a nucleic acid sequence |
  | Σ | 0..1 | integer | Number of linear sequences of amino acids linked through peptide bonds. The number of subunits constituting the SubstanceProtein shall be described. It is possible that the number of subunits can be variable |
  | Σ | 0..* | string | The disulphide bond between two cysteine residues either on the same subunit or on two different subunits shall be described. The position of the disulfide bonds in the SubstanceProtein shall be listed in increasing order of subunit number and position within subunit followed by the abbreviation of the amino acids involved. The disulfide linkage positions shall actually contain the amino acid Cysteine at the respective positions |
  | Σ | 0..* | BackboneElement | This subclause refers to the description of each subunit constituting the SubstanceProtein. A subunit is a linear sequence of amino acids linked through peptide bonds. The Subunit information shall be provided when the finished SubstanceProtein is a complex of multiple sequences; subunits are not used to delineate domains within a single sequence. Subunits are listed in order of decreasing length; sequences of the same length will be ordered by decreasing molecular weight; subunits that have identical sequences will be repeated multiple times |
   | Σ | 0..1 | integer | Index of primary sequences of amino acids linked through peptide bonds in order of decreasing length. Sequences of the same length will be ordered by molecular weight. Subunits that have identical sequences will be repeated and have sequential subscripts |
   | Σ | 0..1 | string | The sequence information shall be provided enumerating the amino acids from N- to C-terminal end using standard single-letter amino acid codes. Uppercase shall be used for L-amino acids and lowercase for D-amino acids. Transcribed SubstanceProteins will always be described using the translated sequence; for synthetic peptide containing amino acids that are not represented with a single letter code an X should be used within the sequence. The modified amino acids will be distinguished by their position in the sequence |
   | Σ | 0..1 | integer | Length of linear sequences of amino acids contained in the subunit |
   | Σ | 0..1 | Attachment | The sequence information shall be provided enumerating the amino acids from N- to C-terminal end using standard single-letter amino acid codes. Uppercase shall be used for L-amino acids and lowercase for D-amino acids. Transcribed SubstanceProteins will always be described using the translated sequence; for synthetic peptide containing amino acids that are not represented with a single letter code an X should be used within the sequence. The modified amino acids will be distinguished by their position in the sequence |
   | Σ | 0..1 | Identifier | Unique identifier for molecular fragment modification based on the ISO 11238 Substance ID |
   | Σ | 0..1 | string | The name of the fragment modified at the N-terminal of the SubstanceProtein shall be specified |
   | Σ | 0..1 | Identifier | Unique identifier for molecular fragment modification based on the ISO 11238 Substance ID |
   | Σ | 0..1 | string | The modification at the C-terminal shall be specified |
 Documentation for this format Documentation for this format  | ||||
See the Extensions for this resource
XML Template
<SubstanceProtein xmlns="http://hl7.org/fhir"><!-- from Resource: id, meta, implicitRules, and language --> <!-- from DomainResource: text, contained, extension, and modifierExtension --> <sequenceType><!-- 0..1 CodeableConcept The SubstanceProtein descriptive elements will only be used when a complete or partial amino acid sequence is available or derivable from a nucleic acid sequence --></sequenceType> <numberOfSubunits value="[integer]"/><!-- 0..1 Number of linear sequences of amino acids linked through peptide bonds. The number of subunits constituting the SubstanceProtein shall be described. It is possible that the number of subunits can be variable --> <disulfideLinkage value="[string]"/><!-- 0..* The disulphide bond between two cysteine residues either on the same subunit or on two different subunits shall be described. The position of the disulfide bonds in the SubstanceProtein shall be listed in increasing order of subunit number and position within subunit followed by the abbreviation of the amino acids involved. The disulfide linkage positions shall actually contain the amino acid Cysteine at the respective positions --> <subunit> <!-- 0..* This subclause refers to the description of each subunit constituting the SubstanceProtein. A subunit is a linear sequence of amino acids linked through peptide bonds. The Subunit information shall be provided when the finished SubstanceProtein is a complex of multiple sequences; subunits are not used to delineate domains within a single sequence. Subunits are listed in order of decreasing length; sequences of the same length will be ordered by decreasing molecular weight; subunits that have identical sequences will be repeated multiple times --> <subunit value="[integer]"/><!-- 0..1 Index of primary sequences of amino acids linked through peptide bonds in order of decreasing length. Sequences of the same length will be ordered by molecular weight. Subunits that have identical sequences will be repeated and have sequential subscripts --> <sequence value="[string]"/><!-- 0..1 The sequence information shall be provided enumerating the amino acids from N- to C-terminal end using standard single-letter amino acid codes. Uppercase shall be used for L-amino acids and lowercase for D-amino acids. Transcribed SubstanceProteins will always be described using the translated sequence; for synthetic peptide containing amino acids that are not represented with a single letter code an X should be used within the sequence. The modified amino acids will be distinguished by their position in the sequence --> <length value="[integer]"/><!-- 0..1 Length of linear sequences of amino acids contained in the subunit --> <sequenceAttachment><!-- 0..1 Attachment The sequence information shall be provided enumerating the amino acids from N- to C-terminal end using standard single-letter amino acid codes. Uppercase shall be used for L-amino acids and lowercase for D-amino acids. Transcribed SubstanceProteins will always be described using the translated sequence; for synthetic peptide containing amino acids that are not represented with a single letter code an X should be used within the sequence. The modified amino acids will be distinguished by their position in the sequence --></sequenceAttachment> <nTerminalModificationId><!-- 0..1 Identifier Unique identifier for molecular fragment modification based on the ISO 11238 Substance ID --></nTerminalModificationId> <nTerminalModification value="[string]"/><!-- 0..1 The name of the fragment modified at the N-terminal of the SubstanceProtein shall be specified --> <cTerminalModificationId><!-- 0..1 Identifier Unique identifier for molecular fragment modification based on the ISO 11238 Substance ID --></cTerminalModificationId> <cTerminalModification value="[string]"/><!-- 0..1 The modification at the C-terminal shall be specified --> </subunit> </SubstanceProtein>
JSON Template
{ "resourceType" : "SubstanceProtein",
// from Resource: id, meta, implicitRules, and language
// from DomainResource: text, contained, extension, and modifierExtension
"sequenceType" : { CodeableConcept }, // The SubstanceProtein descriptive elements will only be used when a complete or partial amino acid sequence is available or derivable from a nucleic acid sequence
"numberOfSubunits" : <integer>, // Number of linear sequences of amino acids linked through peptide bonds. The number of subunits constituting the SubstanceProtein shall be described. It is possible that the number of subunits can be variable
"disulfideLinkage" : ["<string>"], // The disulphide bond between two cysteine residues either on the same subunit or on two different subunits shall be described. The position of the disulfide bonds in the SubstanceProtein shall be listed in increasing order of subunit number and position within subunit followed by the abbreviation of the amino acids involved. The disulfide linkage positions shall actually contain the amino acid Cysteine at the respective positions
"subunit" : [{ // This subclause refers to the description of each subunit constituting the SubstanceProtein. A subunit is a linear sequence of amino acids linked through peptide bonds. The Subunit information shall be provided when the finished SubstanceProtein is a complex of multiple sequences; subunits are not used to delineate domains within a single sequence. Subunits are listed in order of decreasing length; sequences of the same length will be ordered by decreasing molecular weight; subunits that have identical sequences will be repeated multiple times
"subunit" : <integer>, // Index of primary sequences of amino acids linked through peptide bonds in order of decreasing length. Sequences of the same length will be ordered by molecular weight. Subunits that have identical sequences will be repeated and have sequential subscripts
"sequence" : "<string>", // The sequence information shall be provided enumerating the amino acids from N- to C-terminal end using standard single-letter amino acid codes. Uppercase shall be used for L-amino acids and lowercase for D-amino acids. Transcribed SubstanceProteins will always be described using the translated sequence; for synthetic peptide containing amino acids that are not represented with a single letter code an X should be used within the sequence. The modified amino acids will be distinguished by their position in the sequence
"length" : <integer>, // Length of linear sequences of amino acids contained in the subunit
"sequenceAttachment" : { Attachment }, // The sequence information shall be provided enumerating the amino acids from N- to C-terminal end using standard single-letter amino acid codes. Uppercase shall be used for L-amino acids and lowercase for D-amino acids. Transcribed SubstanceProteins will always be described using the translated sequence; for synthetic peptide containing amino acids that are not represented with a single letter code an X should be used within the sequence. The modified amino acids will be distinguished by their position in the sequence
"nTerminalModificationId" : { Identifier }, // Unique identifier for molecular fragment modification based on the ISO 11238 Substance ID
"nTerminalModification" : "<string>", // The name of the fragment modified at the N-terminal of the SubstanceProtein shall be specified
"cTerminalModificationId" : { Identifier }, // Unique identifier for molecular fragment modification based on the ISO 11238 Substance ID
"cTerminalModification" : "<string>" // The modification at the C-terminal shall be specified
}]
}
"resourceType" : "SubstanceProtein",
// from Resource: id, meta, implicitRules, and language
// from DomainResource: text, contained, extension, and modifierExtension
"sequenceType" : { CodeableConcept }, // The SubstanceProtein descriptive elements will only be used when a complete or partial amino acid sequence is available or derivable from a nucleic acid sequence
"numberOfSubunits" : <integer>, // Number of linear sequences of amino acids linked through peptide bonds. The number of subunits constituting the SubstanceProtein shall be described. It is possible that the number of subunits can be variable
"disulfideLinkage" : ["<string>"], // The disulphide bond between two cysteine residues either on the same subunit or on two different subunits shall be described. The position of the disulfide bonds in the SubstanceProtein shall be listed in increasing order of subunit number and position within subunit followed by the abbreviation of the amino acids involved. The disulfide linkage positions shall actually contain the amino acid Cysteine at the respective positions
"subunit" : [{ // This subclause refers to the description of each subunit constituting the SubstanceProtein. A subunit is a linear sequence of amino acids linked through peptide bonds. The Subunit information shall be provided when the finished SubstanceProtein is a complex of multiple sequences; subunits are not used to delineate domains within a single sequence. Subunits are listed in order of decreasing length; sequences of the same length will be ordered by decreasing molecular weight; subunits that have identical sequences will be repeated multiple times
"subunit" : <integer>, // Index of primary sequences of amino acids linked through peptide bonds in order of decreasing length. Sequences of the same length will be ordered by molecular weight. Subunits that have identical sequences will be repeated and have sequential subscripts
"sequence" : "<string>", // The sequence information shall be provided enumerating the amino acids from N- to C-terminal end using standard single-letter amino acid codes. Uppercase shall be used for L-amino acids and lowercase for D-amino acids. Transcribed SubstanceProteins will always be described using the translated sequence; for synthetic peptide containing amino acids that are not represented with a single letter code an X should be used within the sequence. The modified amino acids will be distinguished by their position in the sequence
"length" : <integer>, // Length of linear sequences of amino acids contained in the subunit
"sequenceAttachment" : { Attachment }, // The sequence information shall be provided enumerating the amino acids from N- to C-terminal end using standard single-letter amino acid codes. Uppercase shall be used for L-amino acids and lowercase for D-amino acids. Transcribed SubstanceProteins will always be described using the translated sequence; for synthetic peptide containing amino acids that are not represented with a single letter code an X should be used within the sequence. The modified amino acids will be distinguished by their position in the sequence
"nTerminalModificationId" : { Identifier }, // Unique identifier for molecular fragment modification based on the ISO 11238 Substance ID
"nTerminalModification" : "<string>", // The name of the fragment modified at the N-terminal of the SubstanceProtein shall be specified
"cTerminalModificationId" : { Identifier }, // Unique identifier for molecular fragment modification based on the ISO 11238 Substance ID
"cTerminalModification" : "<string>" // The modification at the C-terminal shall be specified
}]
}
Turtle Template
@prefix fhir: <http://hl7.org/fhir/> .[ a fhir:SubstanceProtein; fhir:nodeRole fhir:treeRoot; # if this is the parser root # from Resource: .id, .meta, .implicitRules, and .language # from DomainResource: .text, .contained, .extension, and .modifierExtension fhir:sequenceType [ CodeableConcept ] ; # 0..1 The SubstanceProtein descriptive elements will only be used when a complete or partial amino acid sequence is available or derivable from a nucleic acid sequence fhir:numberOfSubunits [ integer ] ; # 0..1 Number of linear sequences of amino acids linked through peptide bonds. The number of subunits constituting the SubstanceProtein shall be described. It is possible that the number of subunits can be variable fhir:disulfideLinkage ( [ string ] ... ) ; # 0..* The disulphide bond between two cysteine residues either on the same subunit or on two different subunits shall be described. The position of the disulfide bonds in the SubstanceProtein shall be listed in increasing order of subunit number and position within subunit followed by the abbreviation of the amino acids involved. The disulfide linkage positions shall actually contain the amino acid Cysteine at the respective positions fhir:subunit ( [ # 0..* This subclause refers to the description of each subunit constituting the SubstanceProtein. A subunit is a linear sequence of amino acids linked through peptide bonds. The Subunit information shall be provided when the finished SubstanceProtein is a complex of multiple sequences; subunits are not used to delineate domains within a single sequence. Subunits are listed in order of decreasing length; sequences of the same length will be ordered by decreasing molecular weight; subunits that have identical sequences will be repeated multiple times fhir:subunit [ integer ] ; # 0..1 Index of primary sequences of amino acids linked through peptide bonds in order of decreasing length. Sequences of the same length will be ordered by molecular weight. Subunits that have identical sequences will be repeated and have sequential subscripts fhir:sequence [ string ] ; # 0..1 The sequence information shall be provided enumerating the amino acids from N- to C-terminal end using standard single-letter amino acid codes. Uppercase shall be used for L-amino acids and lowercase for D-amino acids. Transcribed SubstanceProteins will always be described using the translated sequence; for synthetic peptide containing amino acids that are not represented with a single letter code an X should be used within the sequence. The modified amino acids will be distinguished by their position in the sequence fhir:length [ integer ] ; # 0..1 Length of linear sequences of amino acids contained in the subunit fhir:sequenceAttachment [ Attachment ] ; # 0..1 The sequence information shall be provided enumerating the amino acids from N- to C-terminal end using standard single-letter amino acid codes. Uppercase shall be used for L-amino acids and lowercase for D-amino acids. Transcribed SubstanceProteins will always be described using the translated sequence; for synthetic peptide containing amino acids that are not represented with a single letter code an X should be used within the sequence. The modified amino acids will be distinguished by their position in the sequence fhir:nTerminalModificationId [ Identifier ] ; # 0..1 Unique identifier for molecular fragment modification based on the ISO 11238 Substance ID fhir:nTerminalModification [ string ] ; # 0..1 The name of the fragment modified at the N-terminal of the SubstanceProtein shall be specified fhir:cTerminalModificationId [ Identifier ] ; # 0..1 Unique identifier for molecular fragment modification based on the ISO 11238 Substance ID fhir:cTerminalModification [ string ] ; # 0..1 The modification at the C-terminal shall be specified ] ... ) ; ]
Changes since Release 4
| SubstanceProtein |
|
See the Full Difference for further information
Additional definitions: Master Definition XML + JSON, XML Schema/Schematron + JSON Schema, ShEx (for Turtle) + see the extensions, the spreadsheet version & the dependency analysis
Search parameters for this resource. See also the full list of search parameters for this resource, and check the Extensions registry for search parameters on extensions related to this resource. The common parameters also apply. See Searching for more information about searching in REST, messaging, and services.
(No search parameters for this resource)