This page is part of the FHIR Specification (v1.8.0: STU 3 Draft). The current version which supercedes this version is 5.0.0. For a full list of available versions, see the Directory of published versions  . Page versions: R5 R4B R4 R3 R2
. Page versions: R5 R4B R4 R3 R2
Orders and Observations  Work Group Work Group | Maturity Level: 3 | Compartments: Device, Encounter, Patient, Practitioner |
The findings and interpretation of diagnostic tests performed on patients, groups of patients, devices, and locations, and/or specimens derived from these. The report includes clinical context such as requesting and provider information, and some mix of atomic results, images, textual and coded interpretations, and formatted representation of diagnostic reports.
This resource is an event resource from a FHIR workflow perspective - see Workflow. It is the intent of the Orders and Observation Workgroup to align this resource with the workflow pattern for event resources.
A diagnostic report is the set of information that is typically provided by a diagnostic service when investigations are complete. The information includes a mix of atomic results, text reports, images, and codes. The mix varies depending on the nature of the diagnostic procedure, and sometimes on the nature of the outcomes for a particular investigation. In FHIR, the report can be conveyed in a variety of ways including a Document, RESTful API, or Messaging framework. Included within each of these, would be the DiagnosticReport resource itself.
The DiagnosticReport resource has information about the diagnostic report itself, and about the subject and, in the case of lab tests, the specimen of the report. It can also refers to the request details and atomic observations details or image instances. Report conclusions can be expressed as a simple text blob, structured coded data or as an attached fully formatted report such as a PDF.
The DiagnosticReport resource is suitable for the following kinds of diagnostic reports:
The DiagnosticReport resource is not intended to support cumulative result presentation (tabular presentation of past and present results in the resource). The DiagnosticReport resource does not yet provide full support for detailed structured reports of sequencing; this is planned for a future release.
The words "tests", "results", "observations", "panels" and "batteries" are often used interchangeably when describing the various parts of a diagnostic report. This leads to much confusion. The naming confusion is worsened because of the wide variety of forms that the result of a diagnostic investigation can take, as described above. Languages other than English have their own variations on this theme.
This resource uses one particular set of terms. A practitioner "requests" a set of "tests". The diagnostic service returns a "report" which may contain a "narrative" - a written summary of the outcomes, and/or "results" - the individual pieces of atomic data which each are "observations". The results are assembled in "groups" which are nested structures of Observations (traditionally referred to as "panels" or " batteries" by laboratories) that can be used to represent relationships between the individual data items.
Note that many diagnostic processes are procedures that generate observations and diagnostic reports. In many cases, such an observation does not require an explicit representation of the procedure used to create the observation, but where there are details of interest about how the diagnostic procedure was performed, the Procedure resource is used to describe the activity.
In contrast to the Observation resource, the DiagnosticReport resource typically includes additional clinical context and some mix of atomic results, images, imaging reports, textual and coded interpretation, and formatted representations. Laboratory reports, pathology reports, and imaging reports should be represented using the DiagnosticReport resource. The Observation resource is referenced by the DiagnosticReport to provide the atomic results for a particular investigation.
If you have a highly structured report, then use DiagnosticReport - it has data and workflow support. Details about the request for a diagnostic investigation are captured in the various "request" resources (e.g., the DiagnosticRequest) resource and allow the report to connect to clinical workflows. For more narrative driven reports with less work flow (histology/mortuary, etc.), the Composition resource would be more appropriate.
STU Note: The relationship between the two resources is subject to ongoing evaluation during the trial use period.
Feedback is welcome here
.
Image and media representations of the report and supporting images are referenced in the DiagnosticReport resource. The details and actual image instances can be referenced directly in Diagnostic report using the "imaging" element or by indirect reference through the ImagingManifest or ImagingStudy resources which represent the content produced in a DICOM imaging study or set of DICOM Instances of a patient.
This resource is referenced by ClinicalImpression, Condition and Procedure
Structure
| Name | Flags | Card. | Type | Description & Constraints |
|---|---|---|---|---|
 | DomainResource | A Diagnostic report - a combination of request information, atomic results, images, interpretation, as well as formatted reports | ||
  | Σ | 0..* | Identifier | Id for external references to this report |
  | ?!Σ | 1..1 | code | registered | partial | final | corrected | appended | cancelled | entered-in-error DiagnosticReportStatus (Required) |
  | Σ | 0..1 | CodeableConcept | Service category Diagnostic Service Section Codes (Example) |
  | Σ | 1..1 | CodeableConcept | Name/Code for this diagnostic report LOINC Diagnostic Report Codes (Preferred) |
  | Σ | 0..1 | Reference(Patient | Group | Device | Location) | The subject of the report, usually, but not always, the patient |
  | Σ | 0..1 | Reference(Encounter) | Health care event when test ordered |
  | Σ | 0..1 | Clinically Relevant time/time-period for report | |
   | dateTime | |||
  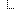 | Period | |||
  | Σ | 0..1 | instant | DateTime this version was released |
  | Σ | 0..* | Reference(Practitioner | Organization) | Responsible Diagnostic Service |
  | 0..* | Reference(DiagnosticRequest | ProcedureRequest | ReferralRequest) | What was requested | |
  | 0..* | Reference(Specimen) | Specimens this report is based on | |
  | 0..* | Reference(Observation) | Observations - simple, or complex nested groups | |
  | 0..* | Reference(ImagingStudy | ImagingManifest) | Reference to full details of imaging associated with the diagnostic report | |
  | Σ | 0..* | BackboneElement | Key images associated with this report |
   | 0..1 | string | Comment about the image (e.g. explanation) | |
  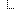 | Σ | 1..1 | Reference(Media) | Reference to the image source |
  | 0..1 | string | Clinical Interpretation of test results | |
  | 0..* | CodeableConcept | Codes for the conclusion SNOMED CT Clinical Findings (Example) | |
 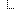 | 0..* | Attachment | Entire report as issued | |
 Documentation for this format Documentation for this format | ||||
UML Diagram (Legend)
XML Template
<DiagnosticReport xmlns="http://hl7.org/fhir"><!-- from Resource: id, meta, implicitRules, and language --> <!-- from DomainResource: text, contained, extension, and modifierExtension --> <identifier><!-- 0..* Identifier Id for external references to this report --></identifier> <status value="[code]"/><!-- 1..1 registered | partial | final | corrected | appended | cancelled | entered-in-error --> <category><!-- 0..1 CodeableConcept Service category --></category> <code><!-- 1..1 CodeableConcept Name/Code for this diagnostic report --></code> <subject><!-- 0..1 Reference(Patient|Group|Device|Location) The subject of the report, usually, but not always, the patient --></subject> <encounter><!-- 0..1 Reference(Encounter) Health care event when test ordered --></encounter> <effective[x]><!-- 0..1 dateTime|Period Clinically Relevant time/time-period for report --></effective[x]> <issued value="[instant]"/><!-- 0..1 DateTime this version was released --> <performer><!-- 0..* Reference(Practitioner|Organization) Responsible Diagnostic Service --></performer> <request><!-- 0..* Reference(DiagnosticRequest|ProcedureRequest|ReferralRequest) What was requested --></request> <specimen><!-- 0..* Reference(Specimen) Specimens this report is based on --></specimen> <result><!-- 0..* Reference(Observation) Observations - simple, or complex nested groups --></result> <imagingStudy><!-- 0..* Reference(ImagingStudy|ImagingManifest) Reference to full details of imaging associated with the diagnostic report --></imagingStudy> <image> <!-- 0..* Key images associated with this report --> <comment value="[string]"/><!-- 0..1 Comment about the image (e.g. explanation) --> <link><!-- 1..1 Reference(Media) Reference to the image source --></link> </image> <conclusion value="[string]"/><!-- 0..1 Clinical Interpretation of test results --> <codedDiagnosis><!-- 0..* CodeableConcept Codes for the conclusion --></codedDiagnosis> <presentedForm><!-- 0..* Attachment Entire report as issued --></presentedForm> </DiagnosticReport>
JSON Template
{ "resourceType" : "DiagnosticReport",
// from Resource: id, meta, implicitRules, and language
// from DomainResource: text, contained, extension, and modifierExtension
"identifier" : [{ Identifier }], // Id for external references to this report
"status" : "<code>", // R! registered | partial | final | corrected | appended | cancelled | entered-in-error
"category" : { CodeableConcept }, // Service category
"code" : { CodeableConcept }, // R! Name/Code for this diagnostic report
"subject" : { Reference(Patient|Group|Device|Location) }, // The subject of the report, usually, but not always, the patient
"encounter" : { Reference(Encounter) }, // Health care event when test ordered
// effective[x]: Clinically Relevant time/time-period for report. One of these 2:
"effectiveDateTime" : "<dateTime>",
"effectivePeriod" : { Period },
"issued" : "<instant>", // DateTime this version was released
"performer" : [{ Reference(Practitioner|Organization) }], // Responsible Diagnostic Service
"request" : [{ Reference(DiagnosticRequest|ProcedureRequest|ReferralRequest) }], // What was requested
"specimen" : [{ Reference(Specimen) }], // Specimens this report is based on
"result" : [{ Reference(Observation) }], // Observations - simple, or complex nested groups
"imagingStudy" : [{ Reference(ImagingStudy|ImagingManifest) }], // Reference to full details of imaging associated with the diagnostic report
"image" : [{ // Key images associated with this report
"comment" : "<string>", // Comment about the image (e.g. explanation)
"link" : { Reference(Media) } // R! Reference to the image source
}],
"conclusion" : "<string>", // Clinical Interpretation of test results
"codedDiagnosis" : [{ CodeableConcept }], // Codes for the conclusion
"presentedForm" : [{ Attachment }] // Entire report as issued
}
"resourceType" : "DiagnosticReport",
// from Resource: id, meta, implicitRules, and language
// from DomainResource: text, contained, extension, and modifierExtension
"identifier" : [{ Identifier }], // Id for external references to this report
"status" : "<code>", // R! registered | partial | final | corrected | appended | cancelled | entered-in-error
"category" : { CodeableConcept }, // Service category
"code" : { CodeableConcept }, // R! Name/Code for this diagnostic report
"subject" : { Reference(Patient|Group|Device|Location) }, // The subject of the report, usually, but not always, the patient
"encounter" : { Reference(Encounter) }, // Health care event when test ordered
// effective[x]: Clinically Relevant time/time-period for report. One of these 2:
"effectiveDateTime" : "<dateTime>",
"effectivePeriod" : { Period },
"issued" : "<instant>", // DateTime this version was released
"performer" : [{ Reference(Practitioner|Organization) }], // Responsible Diagnostic Service
"request" : [{ Reference(DiagnosticRequest|ProcedureRequest|ReferralRequest) }], // What was requested
"specimen" : [{ Reference(Specimen) }], // Specimens this report is based on
"result" : [{ Reference(Observation) }], // Observations - simple, or complex nested groups
"imagingStudy" : [{ Reference(ImagingStudy|ImagingManifest) }], // Reference to full details of imaging associated with the diagnostic report
"image" : [{ // Key images associated with this report
"comment" : "<string>", // Comment about the image (e.g. explanation)
"link" : { Reference(Media) } // R! Reference to the image source
}],
"conclusion" : "<string>", // Clinical Interpretation of test results
"codedDiagnosis" : [{ CodeableConcept }], // Codes for the conclusion
"presentedForm" : [{ Attachment }] // Entire report as issued
}
Turtle Template
@prefix fhir: <http://hl7.org/fhir/> .[ a fhir:DiagnosticReport; fhir:nodeRole fhir:treeRoot; # if this is the parser root # from Resource: .id, .meta, .implicitRules, and .language # from DomainResource: .text, .contained, .extension, and .modifierExtension fhir:DiagnosticReport.identifier [ Identifier ], ... ; # 0..* Id for external references to this report fhir:DiagnosticReport.status [ code ]; # 1..1 registered | partial | final | corrected | appended | cancelled | entered-in-error fhir:DiagnosticReport.category [ CodeableConcept ]; # 0..1 Service category fhir:DiagnosticReport.code [ CodeableConcept ]; # 1..1 Name/Code for this diagnostic report fhir:DiagnosticReport.subject [ Reference(Patient|Group|Device|Location) ]; # 0..1 The subject of the report, usually, but not always, the patient fhir:DiagnosticReport.encounter [ Reference(Encounter) ]; # 0..1 Health care event when test ordered # DiagnosticReport.effective[x] : 0..1 Clinically Relevant time/time-period for report. One of these 2 fhir:DiagnosticReport.effectiveDateTime [ dateTime ] fhir:DiagnosticReport.effectivePeriod [ Period ] fhir:DiagnosticReport.issued [ instant ]; # 0..1 DateTime this version was released fhir:DiagnosticReport.performer [ Reference(Practitioner|Organization) ], ... ; # 0..* Responsible Diagnostic Service fhir:DiagnosticReport.request [ Reference(DiagnosticRequest|ProcedureRequest|ReferralRequest) ], ... ; # 0..* What was requested fhir:DiagnosticReport.specimen [ Reference(Specimen) ], ... ; # 0..* Specimens this report is based on fhir:DiagnosticReport.result [ Reference(Observation) ], ... ; # 0..* Observations - simple, or complex nested groups fhir:DiagnosticReport.imagingStudy [ Reference(ImagingStudy|ImagingManifest) ], ... ; # 0..* Reference to full details of imaging associated with the diagnostic report fhir:DiagnosticReport.image [ # 0..* Key images associated with this report fhir:DiagnosticReport.image.comment [ string ]; # 0..1 Comment about the image (e.g. explanation) fhir:DiagnosticReport.image.link [ Reference(Media) ]; # 1..1 Reference to the image source ], ...; fhir:DiagnosticReport.conclusion [ string ]; # 0..1 Clinical Interpretation of test results fhir:DiagnosticReport.codedDiagnosis [ CodeableConcept ], ... ; # 0..* Codes for the conclusion fhir:DiagnosticReport.presentedForm [ Attachment ], ... ; # 0..* Entire report as issued ]
Changes since DSTU2
| DiagnosticReport | |
| DiagnosticReport.subject | Min Cardinality changed from 1 to 0 |
| DiagnosticReport.effective[x] | Min Cardinality changed from 1 to 0 |
| DiagnosticReport.issued | Min Cardinality changed from 1 to 0 |
| DiagnosticReport.performer |
Min Cardinality changed from 1 to 0 Max Cardinality changed from 1 to * |
| DiagnosticReport.request | Remove Reference(DiagnosticOrder), Add Reference(DiagnosticRequest) |
| DiagnosticReport.imagingStudy | Remove Reference(ImagingObjectSelection), Add Reference(ImagingManifest) |
See the Full Difference for further information
Structure
| Name | Flags | Card. | Type | Description & Constraints |
|---|---|---|---|---|
 | DomainResource | A Diagnostic report - a combination of request information, atomic results, images, interpretation, as well as formatted reports | ||
  | Σ | 0..* | Identifier | Id for external references to this report |
  | ?!Σ | 1..1 | code | registered | partial | final | corrected | appended | cancelled | entered-in-error DiagnosticReportStatus (Required) |
  | Σ | 0..1 | CodeableConcept | Service category Diagnostic Service Section Codes (Example) |
  | Σ | 1..1 | CodeableConcept | Name/Code for this diagnostic report LOINC Diagnostic Report Codes (Preferred) |
  | Σ | 0..1 | Reference(Patient | Group | Device | Location) | The subject of the report, usually, but not always, the patient |
  | Σ | 0..1 | Reference(Encounter) | Health care event when test ordered |
  | Σ | 0..1 | Clinically Relevant time/time-period for report | |
   | dateTime | |||
  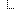 | Period | |||
  | Σ | 0..1 | instant | DateTime this version was released |
  | Σ | 0..* | Reference(Practitioner | Organization) | Responsible Diagnostic Service |
  | 0..* | Reference(DiagnosticRequest | ProcedureRequest | ReferralRequest) | What was requested | |
  | 0..* | Reference(Specimen) | Specimens this report is based on | |
  | 0..* | Reference(Observation) | Observations - simple, or complex nested groups | |
  | 0..* | Reference(ImagingStudy | ImagingManifest) | Reference to full details of imaging associated with the diagnostic report | |
  | Σ | 0..* | BackboneElement | Key images associated with this report |
   | 0..1 | string | Comment about the image (e.g. explanation) | |
   | Σ | 1..1 | Reference(Media) | Reference to the image source |
  | 0..1 | string | Clinical Interpretation of test results | |
  | 0..* | CodeableConcept | Codes for the conclusion SNOMED CT Clinical Findings (Example) | |
  | 0..* | Attachment | Entire report as issued | |
 Documentation for this format Documentation for this format | ||||
XML Template
<DiagnosticReport xmlns="http://hl7.org/fhir"><!-- from Resource: id, meta, implicitRules, and language --> <!-- from DomainResource: text, contained, extension, and modifierExtension --> <identifier><!-- 0..* Identifier Id for external references to this report --></identifier> <status value="[code]"/><!-- 1..1 registered | partial | final | corrected | appended | cancelled | entered-in-error --> <category><!-- 0..1 CodeableConcept Service category --></category> <code><!-- 1..1 CodeableConcept Name/Code for this diagnostic report --></code> <subject><!-- 0..1 Reference(Patient|Group|Device|Location) The subject of the report, usually, but not always, the patient --></subject> <encounter><!-- 0..1 Reference(Encounter) Health care event when test ordered --></encounter> <effective[x]><!-- 0..1 dateTime|Period Clinically Relevant time/time-period for report --></effective[x]> <issued value="[instant]"/><!-- 0..1 DateTime this version was released --> <performer><!-- 0..* Reference(Practitioner|Organization) Responsible Diagnostic Service --></performer> <request><!-- 0..* Reference(DiagnosticRequest|ProcedureRequest|ReferralRequest) What was requested --></request> <specimen><!-- 0..* Reference(Specimen) Specimens this report is based on --></specimen> <result><!-- 0..* Reference(Observation) Observations - simple, or complex nested groups --></result> <imagingStudy><!-- 0..* Reference(ImagingStudy|ImagingManifest) Reference to full details of imaging associated with the diagnostic report --></imagingStudy> <image> <!-- 0..* Key images associated with this report --> <comment value="[string]"/><!-- 0..1 Comment about the image (e.g. explanation) --> <link><!-- 1..1 Reference(Media) Reference to the image source --></link> </image> <conclusion value="[string]"/><!-- 0..1 Clinical Interpretation of test results --> <codedDiagnosis><!-- 0..* CodeableConcept Codes for the conclusion --></codedDiagnosis> <presentedForm><!-- 0..* Attachment Entire report as issued --></presentedForm> </DiagnosticReport>
JSON Template
{ "resourceType" : "DiagnosticReport",
// from Resource: id, meta, implicitRules, and language
// from DomainResource: text, contained, extension, and modifierExtension
"identifier" : [{ Identifier }], // Id for external references to this report
"status" : "<code>", // R! registered | partial | final | corrected | appended | cancelled | entered-in-error
"category" : { CodeableConcept }, // Service category
"code" : { CodeableConcept }, // R! Name/Code for this diagnostic report
"subject" : { Reference(Patient|Group|Device|Location) }, // The subject of the report, usually, but not always, the patient
"encounter" : { Reference(Encounter) }, // Health care event when test ordered
// effective[x]: Clinically Relevant time/time-period for report. One of these 2:
"effectiveDateTime" : "<dateTime>",
"effectivePeriod" : { Period },
"issued" : "<instant>", // DateTime this version was released
"performer" : [{ Reference(Practitioner|Organization) }], // Responsible Diagnostic Service
"request" : [{ Reference(DiagnosticRequest|ProcedureRequest|ReferralRequest) }], // What was requested
"specimen" : [{ Reference(Specimen) }], // Specimens this report is based on
"result" : [{ Reference(Observation) }], // Observations - simple, or complex nested groups
"imagingStudy" : [{ Reference(ImagingStudy|ImagingManifest) }], // Reference to full details of imaging associated with the diagnostic report
"image" : [{ // Key images associated with this report
"comment" : "<string>", // Comment about the image (e.g. explanation)
"link" : { Reference(Media) } // R! Reference to the image source
}],
"conclusion" : "<string>", // Clinical Interpretation of test results
"codedDiagnosis" : [{ CodeableConcept }], // Codes for the conclusion
"presentedForm" : [{ Attachment }] // Entire report as issued
}
"resourceType" : "DiagnosticReport",
// from Resource: id, meta, implicitRules, and language
// from DomainResource: text, contained, extension, and modifierExtension
"identifier" : [{ Identifier }], // Id for external references to this report
"status" : "<code>", // R! registered | partial | final | corrected | appended | cancelled | entered-in-error
"category" : { CodeableConcept }, // Service category
"code" : { CodeableConcept }, // R! Name/Code for this diagnostic report
"subject" : { Reference(Patient|Group|Device|Location) }, // The subject of the report, usually, but not always, the patient
"encounter" : { Reference(Encounter) }, // Health care event when test ordered
// effective[x]: Clinically Relevant time/time-period for report. One of these 2:
"effectiveDateTime" : "<dateTime>",
"effectivePeriod" : { Period },
"issued" : "<instant>", // DateTime this version was released
"performer" : [{ Reference(Practitioner|Organization) }], // Responsible Diagnostic Service
"request" : [{ Reference(DiagnosticRequest|ProcedureRequest|ReferralRequest) }], // What was requested
"specimen" : [{ Reference(Specimen) }], // Specimens this report is based on
"result" : [{ Reference(Observation) }], // Observations - simple, or complex nested groups
"imagingStudy" : [{ Reference(ImagingStudy|ImagingManifest) }], // Reference to full details of imaging associated with the diagnostic report
"image" : [{ // Key images associated with this report
"comment" : "<string>", // Comment about the image (e.g. explanation)
"link" : { Reference(Media) } // R! Reference to the image source
}],
"conclusion" : "<string>", // Clinical Interpretation of test results
"codedDiagnosis" : [{ CodeableConcept }], // Codes for the conclusion
"presentedForm" : [{ Attachment }] // Entire report as issued
}
Turtle Template
@prefix fhir: <http://hl7.org/fhir/> .[ a fhir:DiagnosticReport; fhir:nodeRole fhir:treeRoot; # if this is the parser root # from Resource: .id, .meta, .implicitRules, and .language # from DomainResource: .text, .contained, .extension, and .modifierExtension fhir:DiagnosticReport.identifier [ Identifier ], ... ; # 0..* Id for external references to this report fhir:DiagnosticReport.status [ code ]; # 1..1 registered | partial | final | corrected | appended | cancelled | entered-in-error fhir:DiagnosticReport.category [ CodeableConcept ]; # 0..1 Service category fhir:DiagnosticReport.code [ CodeableConcept ]; # 1..1 Name/Code for this diagnostic report fhir:DiagnosticReport.subject [ Reference(Patient|Group|Device|Location) ]; # 0..1 The subject of the report, usually, but not always, the patient fhir:DiagnosticReport.encounter [ Reference(Encounter) ]; # 0..1 Health care event when test ordered # DiagnosticReport.effective[x] : 0..1 Clinically Relevant time/time-period for report. One of these 2 fhir:DiagnosticReport.effectiveDateTime [ dateTime ] fhir:DiagnosticReport.effectivePeriod [ Period ] fhir:DiagnosticReport.issued [ instant ]; # 0..1 DateTime this version was released fhir:DiagnosticReport.performer [ Reference(Practitioner|Organization) ], ... ; # 0..* Responsible Diagnostic Service fhir:DiagnosticReport.request [ Reference(DiagnosticRequest|ProcedureRequest|ReferralRequest) ], ... ; # 0..* What was requested fhir:DiagnosticReport.specimen [ Reference(Specimen) ], ... ; # 0..* Specimens this report is based on fhir:DiagnosticReport.result [ Reference(Observation) ], ... ; # 0..* Observations - simple, or complex nested groups fhir:DiagnosticReport.imagingStudy [ Reference(ImagingStudy|ImagingManifest) ], ... ; # 0..* Reference to full details of imaging associated with the diagnostic report fhir:DiagnosticReport.image [ # 0..* Key images associated with this report fhir:DiagnosticReport.image.comment [ string ]; # 0..1 Comment about the image (e.g. explanation) fhir:DiagnosticReport.image.link [ Reference(Media) ]; # 1..1 Reference to the image source ], ...; fhir:DiagnosticReport.conclusion [ string ]; # 0..1 Clinical Interpretation of test results fhir:DiagnosticReport.codedDiagnosis [ CodeableConcept ], ... ; # 0..* Codes for the conclusion fhir:DiagnosticReport.presentedForm [ Attachment ], ... ; # 0..* Entire report as issued ]
Changes since DSTU2
| DiagnosticReport | |
| DiagnosticReport.subject | Min Cardinality changed from 1 to 0 |
| DiagnosticReport.effective[x] | Min Cardinality changed from 1 to 0 |
| DiagnosticReport.issued | Min Cardinality changed from 1 to 0 |
| DiagnosticReport.performer |
Min Cardinality changed from 1 to 0 Max Cardinality changed from 1 to * |
| DiagnosticReport.request | Remove Reference(DiagnosticOrder), Add Reference(DiagnosticRequest) |
| DiagnosticReport.imagingStudy | Remove Reference(ImagingObjectSelection), Add Reference(ImagingManifest) |
See the Full Difference for further information
Alternate definitions: Master Definition (XML, JSON), XML Schema/Schematron (for ) + JSON Schema, ShEx (for Turtle), JSON-LD (for RDF as JSON-LD),
| Path | Definition | Type | Reference |
|---|---|---|---|
| DiagnosticReport.status | The status of the diagnostic report as a whole. | Required | DiagnosticReportStatus |
| DiagnosticReport.category | Codes for diagnostic service sections. | Example | Diagnostic Service Section Codes |
| DiagnosticReport.code | Codes that describe Diagnostic Reports. | Preferred | LOINC Diagnostic Report Codes |
| DiagnosticReport.codedDiagnosis | Diagnoses codes provided as adjuncts to the report. | Example | SNOMED CT Clinical Findings |
Examples of nested report groups: the antibody hepatitis order panel code for a group of hepatitis antibody related tests, or the organism code for a group of antibiotic isolate/sensitivities, or a set of perinatal measurements on a single fetus.
If the diagnostic procedure was performed on the patient directly, the effective[x] element is a dateTime, the time it was performed.
If specimens were taken, the clinically relevant time of the report can be derived from the specimen collection times, but
since detailed specimen information is not always available, and nor is the clinically relevant time always exactly the specimen collection time (e.g. complex timed tests), the
reports SHALL always include a effective[x] element. Note that HL7 v2  messages often carry a diagnostically relevant time without carrying any specimen information.
messages often carry a diagnostically relevant time without carrying any specimen information.
ImagingStudy and ImageObjectStudy and the DiagnosticReport.image element are somewhat overlapping - typically, the list of image references in the image element will also be found in one of the imaging study resources. However each caters to different types of displays for different types of purposes. Neither, either, or both may be provided.
Typically, a report is either:all data, no narrative (e.g. Core lab) or a mix of data with some concluding narrative (e.g. Structured Pathology Report, Bone Density), or all narrative (for example a typical imaging report, histopathology). This resource provides for these 3 different presentations:
Note that the conclusion and the coded diagnoses are part of the atomic data, and SHOULD be duplicated in the narrative and in the presented form if the latter is present. The narrative and the presented form serve the same function: a representation of the report for a human. The presented form is included since diagnostic service reports often contain presentation features that are not easy to reproduce in the HTML narrative. Whether or not the presented form is included, the narrative must be a clinically safe view of the diagnostic report; at a minimum, this could be fulfilled by a note indicating that the narrative is not proper representation of the report, and that the presented form must be used, or a generated view from the atomic data. However consumers of the report will best be served if the narrative contains clinically relevant data from the form. Commonly, the following patterns are used:
Note that the nature of reports from the various disciplines that provide diagnostic reports are changing quickly, as expert systems provide improved narrative reporting in high volume reports, structured reporting brings additional data to areas that have classically been narrative based, and the nature of the imaging and laboratory procedures are merging. As a consequence the patterns described above are only examples of how a diagnostic report can be used.
Search parameters for this resource. The common parameters also apply. See Searching for more information about searching in REST, messaging, and services.
| Name | Type | Description | Paths | In Common |
| category | token | Which diagnostic discipline/department created the report | DiagnosticReport.category | |
| code | token | The code for the report as a whole, as opposed to codes for the atomic results, which are the names on the observation resource referred to from the result | DiagnosticReport.code | 8 Resources |
| date | date | The clinically relevant time of the report | DiagnosticReport.effective[x] | 18 Resources |
| diagnosis | token | A coded diagnosis on the report | DiagnosticReport.codedDiagnosis | |
| encounter | reference | The Encounter when the order was made | DiagnosticReport.encounter (Encounter) | 12 Resources |
| identifier | token | An identifier for the report | DiagnosticReport.identifier | 26 Resources |
| image | reference | A reference to the image source. | DiagnosticReport.image.link (Media) | |
| issued | date | When the report was issued | DiagnosticReport.issued | |
| patient | reference | The subject of the report if a patient | DiagnosticReport.subject (Patient) | 31 Resources |
| performer | reference | Who was the source of the report (organization) | DiagnosticReport.performer (Practitioner, Organization) | |
| request | reference | Reference to the test or procedure request. | DiagnosticReport.request (ReferralRequest, ProcedureRequest, DiagnosticRequest) | |
| result | reference | Link to an atomic result (observation resource) | DiagnosticReport.result (Observation) | |
| specimen | reference | The specimen details | DiagnosticReport.specimen (Specimen) | |
| status | token | The status of the report | DiagnosticReport.status | |
| subject | reference | The subject of the report | DiagnosticReport.subject (Group, Device, Patient, Location) |