This page is part of the FHIR Specification (v1.4.0: STU 3 Ballot 3). The current version which supercedes this version is 5.0.0. For a full list of available versions, see the Directory of published versions 

This draft of the CQIF IG is being balloted as the FHIR-Based Clinical Quality Framework (CQF-on-FHIR) as a comment-only ballot for the May 2016 ballot cycle. In addition, this IG will be used to support the SOA/CDS track in the May 2016 FHIR connect-a-thon.
This Implementation Guide is prepared as a Universal Realm Specification with support from the Clinical Quality Framework (CQF) initiative  , which is a public-private partnership sponsored by the Centers for Medicare & Medicaid Services (CMS) and the U.S. Office of the National Coordinator for Health Information Technology (ONC) to harmonize standards for clinical decision support and electronic clinical quality measurement.
, which is a public-private partnership sponsored by the Centers for Medicare & Medicaid Services (CMS) and the U.S. Office of the National Coordinator for Health Information Technology (ONC) to harmonize standards for clinical decision support and electronic clinical quality measurement.

The CQIF Implementation Guide describes a framework for health quality measurement and improvement using Fast Healthcare Interoperability Resources (FHIR). The IG covers a broad range of topics related to healthcare quality in both the decision support and quality measurement domains.
| Topic | Description |
|---|---|
| Overview and Background | If you're interested in the background and development of the Clinical Quality Framework, this topic covers where it came from and why it exists. |
| Representing Knowledge Artifacts | If you want to represent knowledge artifacts such as Event-Condition-Action rules, Order Sets, or Quality Measures, start here. |
| Integrating Decision Support in a Clinical Workflow | If you want to see how to request and respond to clinical guidance, start here. |
| Sharing Knowledge Artifacts | If you want to distribute knowledge artifacts, start here. |
| Quality Reporting | If you want to define or report clinical quality measures, start here. |

The CQIF Implementation Guide is sponsored by the Clinical Decision Support (CDS) and Clinical Quality Information (CQI) HL7 Work Groups, with input and coordination from the FHIR Infrastructure and Service Oriented Architecture HL7 Work Groups.
The Clinical Quality Framework initiative has focused on harmonizing the historically disjointed specifications used by the Clinical Quality Measurement and Clinical Decision Support communities. Specifically, the initiative has focused on the specifications used to represent knowledge artifacts within the two communities. The strategy employed has been to break the conceptual content of knowledge artifacts into three core components:
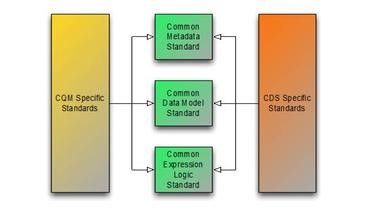
The first component has resulted in the Clinical Quality Common Metadata Conceptual Model  , an informative document harmonizing metadata requirements between Quality Measurement and Decision Support artifacts.
, an informative document harmonizing metadata requirements between Quality Measurement and Decision Support artifacts.
The second component has resulted in the QUICK Conceptual  and Logical Models, a harmonization of the Virtual Medical Record (vMR)
and Logical Models, a harmonization of the Virtual Medical Record (vMR)  used in Decision Support and the Quality Data Model (QDM)
used in Decision Support and the Quality Data Model (QDM)  used in Quality Measurement, and realized in FHIR as the QICore profiles.
used in Quality Measurement, and realized in FHIR as the QICore profiles.
And finally, the third component has resulted in the Clinical Quality Language Specification  , a harmonization of the expressive capabilities of the Clinical Decision Support Knowledge Artifact Specification (CDS KAS)
, a harmonization of the expressive capabilities of the Clinical Decision Support Knowledge Artifact Specification (CDS KAS)  (produced by the Health eDecisions (HeD) S&I Framework Initiative), and the Health Quality Measures Format (HQMF)
(produced by the Health eDecisions (HeD) S&I Framework Initiative), and the Health Quality Measures Format (HQMF)  .
.
As part of the ongoing CQF initiative pilot efforts, these developing specifications are being used to support knowledge artifact sharing, as well as evaluation of knowledge artifacts as part of decision support request/response and measure evaluation.
This implementation guide continues the harmonization of quality domain specifications by defining an approach to using a FHIR server as a component of a knowledge system in one of two roles:
As such, this implementation guide references resources in the base specification, as well as defines profiles that support multiple use cases in the clinical quality improvement and measurement space. From the perspective of a Knowledge Author, this IG specifies the approach to representing knowledge artifacts within FHIR.
From the perspective of a Knowledge Content Provider, this IG defines search functionality for using a FHIR server as a knowledge artifact repository.
From the perspective of a Knowledge Service Provider, this IG defines operations and profiles in support of evaluating quality measures, and defining a service for guidance request and response, consistent with the approach taken by the current Decision Support Service specification.
And finally, from the perspective of a Knowledge Service Consumer, this IG defines the expected available operations and behavior of a knowledge service.

This implementation guide focuses on several primary use cases:
The sharing use case is the focus of the Representing Knowledge Artifacts topic, which covers how to use the various types and resources defined within FHIR to represent computable knowledge artifacts such as Event-Condition-Action rules, Documentation Templates, Order Sets, and Quality Measures.
The evaluation use case is discussed in the next three topics, Integrating Decision Support in a Clinical Workflow, Using a Documentation Template in a Clinical Workflow, and Using an OrderSet in a Clinical Workflow, which cover how to use the various decision support artifacts in a clinical workflow.
The distribution use case is discussed in the Sharing Knowledge Artifacts topic, which covers how to use the Search Parameters defined for each of the resources to expose and retrieve knowledge artifacts.
And finally, Quality Reporting is covered in a separate topic focused on how to use the Measure and MeasureReport resources to define and report on quality measures.

The keywords SHALL, SHOULD, MAY, NEED NOT, SHOULD NOT, and SHALL NOT in this document are to be interpreted as described in the HL7 Version 3 Publishing Facilitator's Guide.

The approach and representations within this guide are intended to be consistent with the following specifications:





For this ballot cycle, we are presenting this implementation guide as a comment-only ballot with the objective of gathering community feedback. We welcome any comments, criticisms and suggestions on any topic, but in particular, we seek feedback on the following areas:

This material includes SNOMED Clinical Terms ® (SNOMED CT®), which are used by permission of the International Health Terminology Standards Development Organization (IHTSDO). All rights reserved. SNOMED CT was originally created by The College of American Pathologists. "SNOMED ®" and "SNOMED CT ®" are registered trademarks of the IHTSDO.
This material contains content from LOINC® (http://loinc.org  ). The LOINC table, LOINC codes, and LOINC panels and forms file are copyright © 1995-2011, Regenstrief Institute, Inc. and the Logical Observation Identifiers Names and Codes (LOINC) Committee and available at no cost under the license at http://loinc.org/terms-of-use
). The LOINC table, LOINC codes, and LOINC panels and forms file are copyright © 1995-2011, Regenstrief Institute, Inc. and the Logical Observation Identifiers Names and Codes (LOINC) Committee and available at no cost under the license at http://loinc.org/terms-of-use  .
.
This material contains content from the Unified Code for Units of Measure (UCUM) (http://unitsofmeasure.org  ). The UCUM specification is copyright © 1999-2013, Regenstrief Institute, Inc. and available at no cost under the license at http://unitsofmeasure.org/trac/wiki/TermsOfUse
). The UCUM specification is copyright © 1999-2013, Regenstrief Institute, Inc. and available at no cost under the license at http://unitsofmeasure.org/trac/wiki/TermsOfUse  .
.
This material contains quality measure content developed by the National Committee for Quality Assurance (NCQA). The measure content is copyright (c) 2008-2013 National Committee for Quality Assurance and used in accordance with the NCQA license terms for non-commercial use.

| Project Facilitator/Editor | Bryn Rhodes | Database Consulting Group | bryn@databaseconsultinggroup.com |
| Project Facilitator/Editor | Jason Walonoski | The MITRE Corporation | jwalonoski@mitre.org |
| Editor | Marc Hadley | The MITRE Corporation | mhadley@mitre.org |
| Modeling Facilitator | Gay Dolin | Intelligent Medical Objects | gdolin@imo-online.com |
| Publishing Facilitator | Lloyd McKenzie | Gevity | lmckenzie@gevityinc.com |
| Domain Expert | KP Sethi | Lantana Consulting Group | kp.sethi@lantanagroup.com |
| Domain Expert | Chris Moesel | The MITRE Corporation | cmoesel@mitre.org |
| Domain Expert | Darrell Woelk | SocialCare | dwoelk@socialcare.com |
| Vocabulary Facilitator | Sarah Ryan | The MITRE Corporation | sarahryan@mitre.org |
| Vocabulary Facilitator | Rob McClure | MD Partners | rmcclure@mdpartners.com |
| Co-Chair | Crystal Kallem | Lantana Consulting Group | crystal.kallem@lantanagroup.com |
| Co-Chair | Patricia Craig | The Joint Commission | pcraig@jointcommission.org |
| Co-Chair | Floyd Eisenberg | iParsimony LLC | feisenberg@iparsimony.com |
| Co-Chair | Chris Millet | National Quality Forum | cmillet@qualityforum.org |
| Co-Chair | Walter Suarez | Kaiser Permanente | walter.g.suarez@kp.org |
| Co-Chair | Guilherme Del Fiol | University of Utah Health Care | guilherme.delfiol@utah.edu |
| Co-Chair | Ken Kawamoto | University of Utah Health Care | kensaku.kawamoto@utah.edu |
| Co-Chair | Robert Jenders | Charles Drew University/UCLA | jenders@ucla.edu |
| Co-Chair | Howard Strasberg | Wolters Kluwer Health | howard.strasberg@wolterskluwer.com |