This page is part of the FHIR Specification (v0.5.0: DSTU 2 Ballot 2). The current version which supercedes this version is 5.0.0. For a full list of available versions, see the Directory of published versions 

This resource maintained by the Clinical Decision Support Work Group
Indicates an actual or potential clinical issue with or between one or more active or proposed clinical actions for a patient. E.g. Drug-drug interaction, Ineffective treatment frequency, Procedure-condition conflict, etc.

This resource applies to various circumstances where there is a concern about an existing or proposed set of clinical activity. The issue could relate to multiple actions/proposed actions or could be specific to a single action. It does not apply to administrative issues (e.g. lack of user permissions) but could relate to violation of patient consent limitations. Examples include:
This resource is not intended to be used in defining general prohibitions on actions such as "No NSAIDs", "No solid oral dose forms" or "No MRIs - metalic tatoos". These guidelines can be captured using the AllergyIntolerance, and/or Flag resources. Similarly this resource is not to be used to capture clinical facts that may imply contraindications such as pregnancy, breast feeding, patient preferences, past procedures, etc. These would be represented using Condition, Procedure or other resources.

This resources only applies to documenting a risk associated with a specific planned or ongoing action, not a general propensity to risk. The latter would be handled using AllergyIntolerance for substance-specific issues or Flag for other types of issues.
This resource is limited to clinical issues associated with a proposed or ongoing action. It does not cover business/technical issues such as lack of permission, duplicate identifiers and other business rule violations. Business and technical issues are conveyed using the OperationOutcome resource. It is possible to have both OperationOutcome and Contraindication together, where the OperationOutcome might indicate that a requested action was rejected due to a clinical issue and the Contraindication provides the details of the issue.

Contraindications are typically identified by decision support systems. However, they may also be captured directly by clinicians. The latter typically happens for one of two reasons:
Decision-support generated contraindications can result from calling a decision-support engine directly (e.g. via a custom OperationDefinition) or as part of an attempt to perform some other function (creating an order, submitting an insurance claim, capturing a medication list). When the contraindications are generated as a byproduct of performing some other sort of action, they may be included in the "response" to the requested action in the same manner as an OperationOutcome. In fact, both may be present - the OperationOutcome indicating that there was a warning or error associated with the request and a Contraindication providing the clinical details. (The OperationOutcome could point to the Contraindication via an extension.)
In those circumstances where requested operations are rejected as a result of a contraindication, the workflow may support allowing the operation to be re-tried provided the identified contraindication is included as part of the submission (possibly also including a mitigation). In doing so, the sender acknowledges the contraindication and takes responsibility for it, thus allowing the requested operation to proceed. See Linking to Contraindications for guidance on how a Contraindication instance might be included as part of another operation.
Systems that require such workflows should document expected behavior as part of their Conformance declarations.

Structure
| Name | Flags | Card. | Type | Description & Constraints |
|---|---|---|---|---|
 | DomainResource | Clinical issue with action | ||
  | Σ | 0..1 | Patient | Associated patient |
  | Σ | 0..1 | CodeableConcept | E.g. Drug-drug, duplicate therapy, etc. ContraindicationCategory (Required) |
  | Σ | 0..1 | code | high | medium | low ContraindicationSeverity (Required) |
  | Σ | 0..* | Any | Problem resource |
  | 0..1 | string | Description and context | |
  | Σ | 0..1 | dateTime | When identified |
  | Σ | 0..1 | Practitioner | Device | Who found issue? |
  | Σ | 0..1 | Identifier | Unique id for the contraindication |
  | 0..1 | uri | Authority for issue | |
 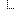 | 0..* | Element | Step taken to address | |
   | 1..1 | CodeableConcept | What mitigation? ContraindicationMitigationAction (Required) | |
   | 0..1 | dateTime | Date committed | |
  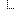 | 0..1 | Practitioner | Who is committing? |
UML Diagram
XML Template
<Contraindication xmlns="http://hl7.org/fhir"><!-- from Resource: id, meta, implicitRules, and language --> <!-- from DomainResource: text, contained, extension, and modifierExtension --> <patient><!-- 0..1 Reference(Patient) Associated patient --></patient> <category><!-- 0..1 CodeableConcept E.g. Drug-drug, duplicate therapy, etc. --></category> <severity value="[code]"/><!-- 0..1 high | medium | low --> <implicated><!-- 0..* Reference(Any) Problem resource --></implicated> <detail value="[string]"/><!-- 0..1 Description and context --> <date value="[dateTime]"/><!-- 0..1 When identified --> <author><!-- 0..1 Reference(Practitioner|Device) Who found issue? --></author> <identifier><!-- 0..1 Identifier Unique id for the contraindication --></identifier> <reference value="[uri]"/><!-- 0..1 Authority for issue --> <mitigation> <!-- 0..* Step taken to address --> <action><!-- 1..1 CodeableConcept What mitigation? --></action> <date value="[dateTime]"/><!-- 0..1 Date committed --> <author><!-- 0..1 Reference(Practitioner) Who is committing? --></author> </mitigation> </Contraindication>
JSON Template
{ "resourceType" : "Contraindication",
// from Resource: id, meta, implicitRules, and language
// from DomainResource: text, contained, extension, and modifierExtension
"patient" : { Reference(Patient) }, // Associated patient
"category" : { CodeableConcept }, // E.g. Drug-drug, duplicate therapy, etc.
"severity" : "<code>", // high | medium | low
"implicated" : [{ Reference(Any) }], // Problem resource
"detail" : "<string>", // Description and context
"date" : "<dateTime>", // When identified
"author" : { Reference(Practitioner|Device) }, // Who found issue?
"identifier" : { Identifier }, // Unique id for the contraindication
"reference" : "<uri>", // Authority for issue
"mitigation" : [{ // Step taken to address
"action" : { CodeableConcept }, // R! What mitigation?
"date" : "<dateTime>", // Date committed
"author" : { Reference(Practitioner) } // Who is committing?
}]
}
"resourceType" : "Contraindication",
// from Resource: id, meta, implicitRules, and language
// from DomainResource: text, contained, extension, and modifierExtension
"patient" : { Reference(Patient) }, // Associated patient
"category" : { CodeableConcept }, // E.g. Drug-drug, duplicate therapy, etc.
"severity" : "<code>", // high | medium | low
"implicated" : [{ Reference(Any) }], // Problem resource
"detail" : "<string>", // Description and context
"date" : "<dateTime>", // When identified
"author" : { Reference(Practitioner|Device) }, // Who found issue?
"identifier" : { Identifier }, // Unique id for the contraindication
"reference" : "<uri>", // Authority for issue
"mitigation" : [{ // Step taken to address
"action" : { CodeableConcept }, // R! What mitigation?
"date" : "<dateTime>", // Date committed
"author" : { Reference(Practitioner) } // Who is committing?
}]
}
Structure
| Name | Flags | Card. | Type | Description & Constraints |
|---|---|---|---|---|
 | DomainResource | Clinical issue with action | ||
  | Σ | 0..1 | Patient | Associated patient |
  | Σ | 0..1 | CodeableConcept | E.g. Drug-drug, duplicate therapy, etc. ContraindicationCategory (Required) |
  | Σ | 0..1 | code | high | medium | low ContraindicationSeverity (Required) |
  | Σ | 0..* | Any | Problem resource |
  | 0..1 | string | Description and context | |
  | Σ | 0..1 | dateTime | When identified |
  | Σ | 0..1 | Practitioner | Device | Who found issue? |
  | Σ | 0..1 | Identifier | Unique id for the contraindication |
  | 0..1 | uri | Authority for issue | |
 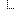 | 0..* | Element | Step taken to address | |
   | 1..1 | CodeableConcept | What mitigation? ContraindicationMitigationAction (Required) | |
   | 0..1 | dateTime | Date committed | |
  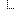 | 0..1 | Practitioner | Who is committing? |
XML Template
<Contraindication xmlns="http://hl7.org/fhir"><!-- from Resource: id, meta, implicitRules, and language --> <!-- from DomainResource: text, contained, extension, and modifierExtension --> <patient><!-- 0..1 Reference(Patient) Associated patient --></patient> <category><!-- 0..1 CodeableConcept E.g. Drug-drug, duplicate therapy, etc. --></category> <severity value="[code]"/><!-- 0..1 high | medium | low --> <implicated><!-- 0..* Reference(Any) Problem resource --></implicated> <detail value="[string]"/><!-- 0..1 Description and context --> <date value="[dateTime]"/><!-- 0..1 When identified --> <author><!-- 0..1 Reference(Practitioner|Device) Who found issue? --></author> <identifier><!-- 0..1 Identifier Unique id for the contraindication --></identifier> <reference value="[uri]"/><!-- 0..1 Authority for issue --> <mitigation> <!-- 0..* Step taken to address --> <action><!-- 1..1 CodeableConcept What mitigation? --></action> <date value="[dateTime]"/><!-- 0..1 Date committed --> <author><!-- 0..1 Reference(Practitioner) Who is committing? --></author> </mitigation> </Contraindication>
JSON Template
{ "resourceType" : "Contraindication",
// from Resource: id, meta, implicitRules, and language
// from DomainResource: text, contained, extension, and modifierExtension
"patient" : { Reference(Patient) }, // Associated patient
"category" : { CodeableConcept }, // E.g. Drug-drug, duplicate therapy, etc.
"severity" : "<code>", // high | medium | low
"implicated" : [{ Reference(Any) }], // Problem resource
"detail" : "<string>", // Description and context
"date" : "<dateTime>", // When identified
"author" : { Reference(Practitioner|Device) }, // Who found issue?
"identifier" : { Identifier }, // Unique id for the contraindication
"reference" : "<uri>", // Authority for issue
"mitigation" : [{ // Step taken to address
"action" : { CodeableConcept }, // R! What mitigation?
"date" : "<dateTime>", // Date committed
"author" : { Reference(Practitioner) } // Who is committing?
}]
}
"resourceType" : "Contraindication",
// from Resource: id, meta, implicitRules, and language
// from DomainResource: text, contained, extension, and modifierExtension
"patient" : { Reference(Patient) }, // Associated patient
"category" : { CodeableConcept }, // E.g. Drug-drug, duplicate therapy, etc.
"severity" : "<code>", // high | medium | low
"implicated" : [{ Reference(Any) }], // Problem resource
"detail" : "<string>", // Description and context
"date" : "<dateTime>", // When identified
"author" : { Reference(Practitioner|Device) }, // Who found issue?
"identifier" : { Identifier }, // Unique id for the contraindication
"reference" : "<uri>", // Authority for issue
"mitigation" : [{ // Step taken to address
"action" : { CodeableConcept }, // R! What mitigation?
"date" : "<dateTime>", // Date committed
"author" : { Reference(Practitioner) } // Who is committing?
}]
}
Alternate definitions: Schema/Schematron, Resource Profile (XML, JSON)

| Path | Definition | Type | Reference |
|---|---|---|---|
| Contraindication.category | Codes identifying the general type of contraindication. E.g. Drug-drug interaction, Timing issue, Duplicate therapy, etc. | Required | http://hl7.org/fhir/vs/contraindication-category |
| Contraindication.severity | Indicates the potential degree of impact of the identified issue on the patient | Required | http://hl7.org/fhir/v3/vs/SeverityObservation |
| Contraindication.mitigation.action | Codes describing steps taken to resolve the contraindication or other circumstances that mitigate the risk associated with the contraindication. E.g. 'added concurrent therapy', 'prior therapy documented', etc. | Required | http://hl7.org/fhir/vs/contraindication-mitigation-action |

Contraindication follows the pattern of linking from the resource created "second". As Contraindication originates in response to one or more other existing records, it points to those records rather than being pointed to from them.
In some cases, a contraindication might be associated with a single record. When this occurs, it may be stored as a contained resource within the implicated resource provided that there is no expected need to search for the contraindication directly. However, with contraindications that implicate multiple records, containment is more problematic. In some workflows, a contraindication might be deemed to be "owned" by the record whose creation triggers the contrindication being created - i.e. the "second" or "last" record. However, where multiple actions are proposed as part of a single submission, there can be no single owner and containment will not be feasible.
If there is a strong need to point from an implicated resource to Contraindication and containment is not appropriate, an extension can be used.


Search parameters for this resource. The common parameters also apply. See Searching for more information about searching in REST, messaging, and services.
| Name | Type | Description | Paths |
| author | reference | Who found issue? | Contraindication.author (Device, Practitioner) |
| category | token | E.g. Drug-drug, duplicate therapy, etc. | Contraindication.category |
| date | date | When identified | Contraindication.date |
| identifier | token | Unique id for the contraindication | Contraindication.identifier |
| implicated | reference | Problem resource | Contraindication.implicated (Any) |
| patient | reference | Associated patient | Contraindication.patient (Patient) |