This page is part of the Retrieval of Real World Data for Clinical Research (v1.0.0-ballot: STU1 Ballot 1) based on FHIR (HL7® FHIR® Standard) R4. The current version which supersedes this version is 1.0.0. For a full list of available versions, see the Directory of published versions
| Official URL: http://hl7.org/fhir/uv/vulcan-rwd/ImplementationGuide/hl7.fhir.uv.vulcan-rwd | Version: 1.0.0-ballot | |||
| Draft as of 2022-12-04 | Computable Name: RealWorldData | |||
In the context of clinical research, Real World Data (RWD) are data created in the “real world” of everyday experience, such as a routine patient visit to a healthcare provider, as opposed to data created under clearly defined protocols typical of controlled clinical trials. The primary purpose for such data, collected for a purpose other than use in a clinical trial, is in support of clinical care of patients and knowledge for their healthcare providers. However, large amounts of such information could potentially be used for the secondary purpose of supporting clinical research to analyze the data and generate supporting evidence for, as an example, a new indicated use for an already approved pharmaceutical drug or safety-related analyses. The identification of outcomes, safety signals, or other research/regulatory insights demonstrated with the use of RWD is commonly called Real World Evidence (RWE).
The main goal of this FHIR Implementation Guide is to help define a minimal set of clinical research FHIR resources and elements in an EHR that can be utilized in an interoperable and consistent manner for clinical research objectives (e.g., academic, regulatory, etc.). This Guide defines FHIR profiles that can be used in an EHR setting to represent data that will be supportive of RWD research needs and also to retrieve relevant research data from Real World Data (RWD) sources to facilitate downstream use (directly or after transformation) for submissions to pharmaceutical regulatory agencies. The downstream use of retrieved data is not covered in this guide but separate efforts exist to give advice for such use (for example, the FHIR to CDISC Joint Mapping Implementation Guide.
Many sources of RWD exist, but for the current phase of work, the scope of this Implementation Guide is limited to the use of Electronic Health Record (EHR) systems as sources of RWD. The intent is for future iterations of the Implementation Guide to have a wider scope of RWD such as from Registries, Payer systems, and HIEs. Additionally, our focus is currently limited to the use of EHR data for retrospective analysis of data which already exists as part of normal healthcare encounters - not data created as part of prospective clinical studies.
We are very aware that the use of EHRs as a mode of direct data collections for traditional prospective clinical trials (sometimes called “electronic source data” or “eSource” activities) is also of great interest. While this is not currently in scope of this implementation guide, we consider it highly likely that types of solutions developed for eSource and for RWD will have significant overlap
This guide is dependent on the International Patient Summary (IPS) project for a baseline dataset from which to build its profiles. The profiles built will enhance the IPS profiles for the purposes of conveying data needed for clinical research. IPS was chosen over a regional guide like US Core because it is a more generalized representation of data and has less constraints than regional guides. To that end, a guide that bases itself on IPS will be more highly interoperable but can be further constrained to be in line with a regional guide like US Core, Australia Core, or Canadian Core.
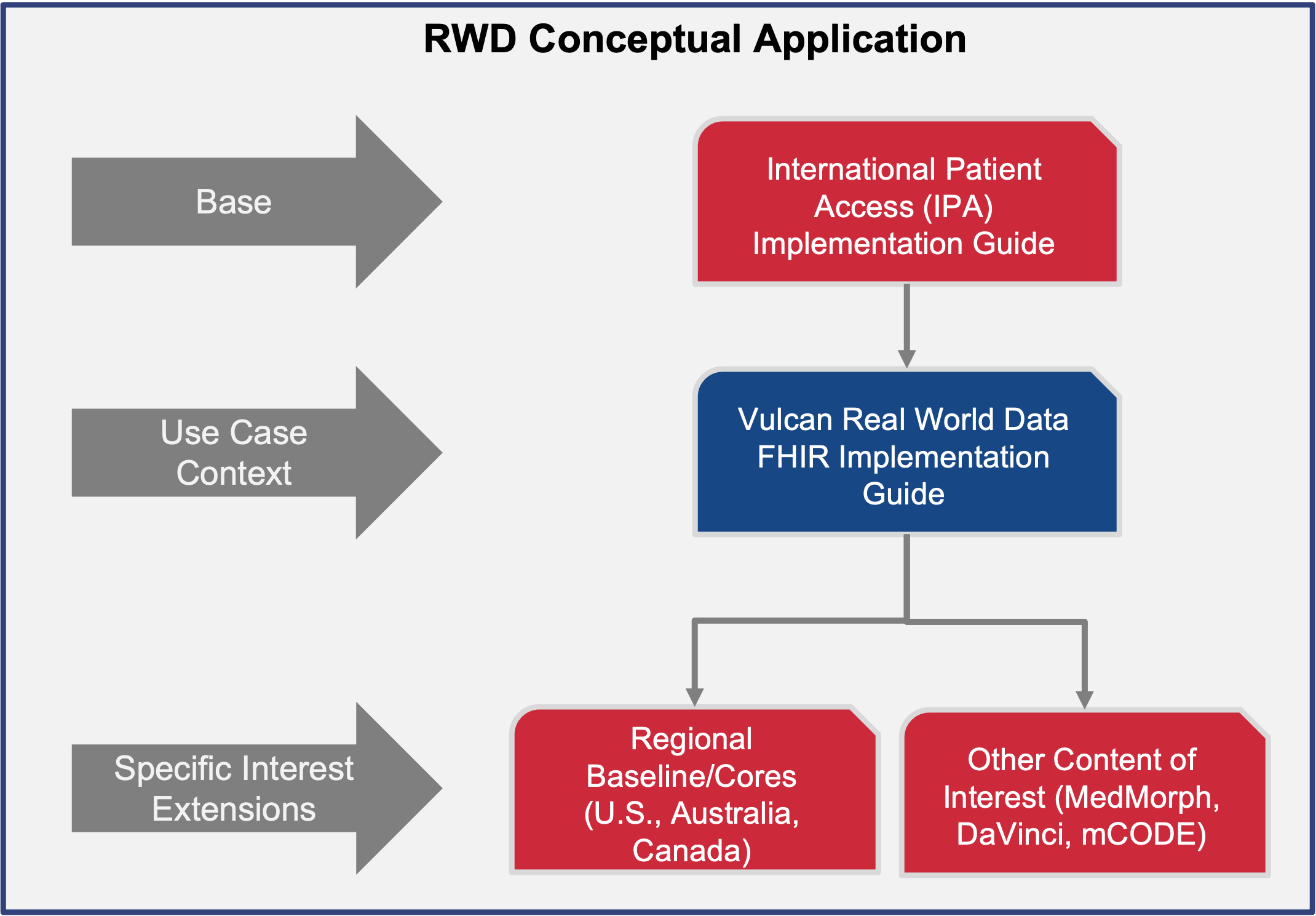
This page has more information on the relationship between this guide and other foundational guides.
This guide defines the FHIR building blocks to meet use cases which will eventually mature the minimal set of common resources and elements. It is being developed using an iterative use case approach, identifying the minimal set of EHR-based information needed to answer a small set of research questions and creating a set of FHIR profiles for representing this needed information in an EHR, then repeating the process with a new question. As more use cases are considered, more common resources and elements will be added to the guide. It provides an opportunity to help establish future US Core, Australia Core, Japan Core, etc. as they wish to scale their guides and profiles. The mappings to achieve different outcomes are dependent on other projects (eg. FHIR to CDISC, FHIR to OMOP, etc.).
The Vulcan Real World Data project determined that there are two phases to requesting data from an EHR:
The first phase is building a cohort by querying for patients based on a set of inclusion and exclusion critieria. Although these criteria may be represented as FHIR objects, this guide does not include a process from converting the criteria expressed as FHIR instances into queries against an EHR. Instead it presumes that the needed healthcare data is known by the requesting software in some manner. In this phase, a number of queries are made against an EHR to narrow down the set of patients to those who meet the research study criteria. A set of patient identifiers is retrieved and becomes input into the next phase of requesting data. NOTE: This phase may not be necessary if the set of patients is already known for a study. If the proper patient identifiers are known, it is possible to skip this phase and go directly to retrieving healthcare data for the known patients. For more information about this phase, see Cohort Building Phase.
After specific patients have been determined, the next phase is to retrieve their healthcare data. This guide inherits from the International Patient Summary and uses the IPS as the means to transfer healthcare data. There are two means to retrieve the summary:
For IPS-Capable EHR systems, a request to retrieve a specific patient’s IPS will be made. The IPS that is returned will be enhanced by using the profiles defined in this guide. It will in effect be a “Study-Enhanced IPS”. The EHR will receive a request using FHIR APIs with the patient’s identifier as a parameter and will return the Study-Enhanced IPS as the payload. For information on what the Study-Enhanced IPS contains, see Retrieve Study Enhanced IPS.
For EHRs that do not have the ability to package and return IPS documents, a set of standard FHIR queries have been determined. EHRs will have to support the specific search parameters needed to find healthcare data for a specific patient. The CTMS or a downstream agent will make all of the queries and will then package all of the returned information into the Study-Enhanced IPS. For information on the needed queries, see Individual EHR Queries.
To properly implement this guide, there are a set of technical considerations besides the specific queries and profiles defined. See Technical Considerations for more details.