| LinkId | Text | Definition | Answer |
|---|
  questionnaireresponse-sdc-profile-example-loinc questionnaireresponse-sdc-profile-example-loinc | | QuestionnaireResponse | |
   Medication/header Medication/header | | | |
 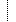 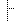  0 0 | Form ID: | | 000 |
 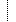 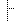  74080-3/74081-1 74080-3/74081-1 | Event ID: | | 456 |
 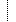 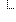  74080-3/30947-6 74080-3/30947-6 | Initial Report Date (HERF Q1) | | 2016-03-14 |
 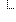  Medication/SEC00 Medication/SEC00 | Medication or Other Substance | | |
    Medication/SEC01/74080-3 Medication/SEC01/74080-3 | | | |
     74080-3/74076-1 74080-3/74076-1 | What type of medication/substance was involved? | | LOINC LA20271-5: Medications |
      74080-3/74075-3 74080-3/74075-3 | What type of medication? | | LOINC LA20278-0: Prescription or over-the-counter (including herbal supplements) |
     74080-3/74072-0 74080-3/74072-0 | Which of the following best characterizes the event? | | LOINC LA20275-6: Incorrect action (process failure or error) (e.g., such as administering overdose or incorrect medication) |
     74080-3/74071-2 74080-3/74071-2 | What was the incorrect action? | | LOINC LA20276-4: Incorrect patient |
     74080-3/74063-9 74080-3/74063-9 | At what stage in the process did the event originate, regardless of the stage at which it was discovered? | | LOINC LA20296-2: Administering |
    74080-3/74078-7 74080-3/74078-7 | | | |
     74080-3/74078-7/74062-1 74080-3/74078-7/74062-1 | Generic name or investigational drug name | | Acetaminophen |
     74080-3/74078-7/74055-5 74080-3/74078-7/74055-5 | Dosage form of Product | | Tablet |
     74080-3/74078-7/74053-0 74080-3/74078-7/74053-0 | Was this medication/substance prescribed for this patient? | | LOINC LA32-8: No |
     Medication/74052-2 Medication/74052-2 | Was this medication/substance given to this patient? | | LOINC LA33-6: Yes |
 Documentation for this format Documentation for this format |


































 Documentation for this format
Documentation for this format