This page is part of the HL7 Laboratory Report (v1.0.0-ballot: STU 1 Ballot 1) based on FHIR (HL7® FHIR® Standard) R4. No current official version has been published yet. For a full list of available versions, see the Directory of published versions
| Page standards status: Informative |
There are two ways - in principle - for representing a Laboratory report in HL7 FHIR:
The solution proposed in this guide tries to take in consideration the very different meanings that a Laboratory Report can assume in different countries.
In fact, in many countries a Laboratory Report is a legally signed document. Reports are often structured in sections and may include different kinds of test results. There are several implementations still based on HL7 CDA (mainly IHE XD-LAB) that are progressivly moving to HL7 FHIR and are still using document exchange infrastructures (e.g. IHE XD*).
In others, a report is a simple collection of results, not treated as a document and often not providing any structured content.
This guide proposes a scalable approach:
These choices would allow for different jurisdictions to select the solution that better fits with their requirements, while assuring the capability for everyone to retrieve laboratory report data by searching via DiagnosticReport.
In brief:
The following figures graphically summarizes the described design approach.
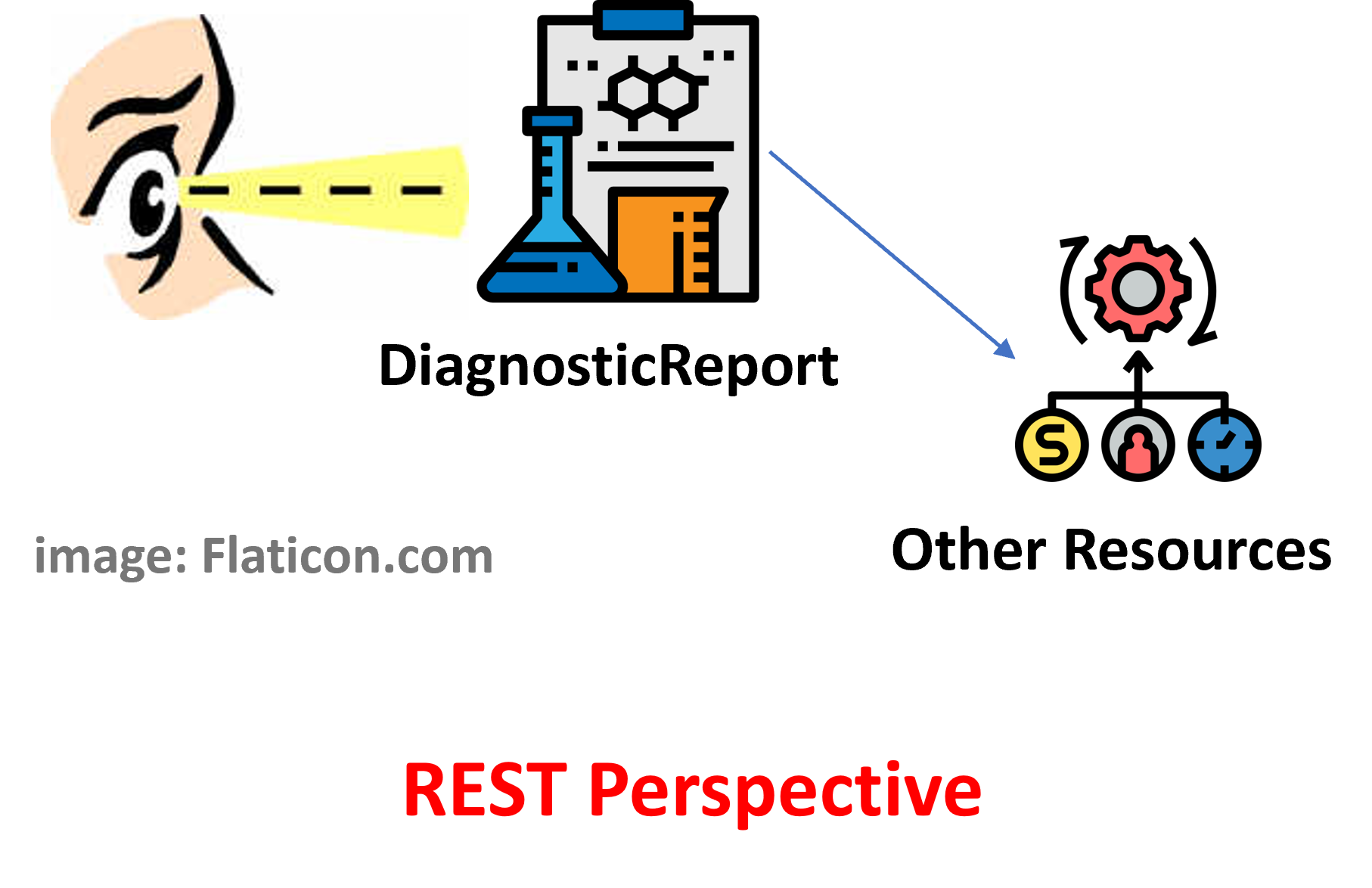
Figure 1 - Report as collection of results: DiagnosticReport
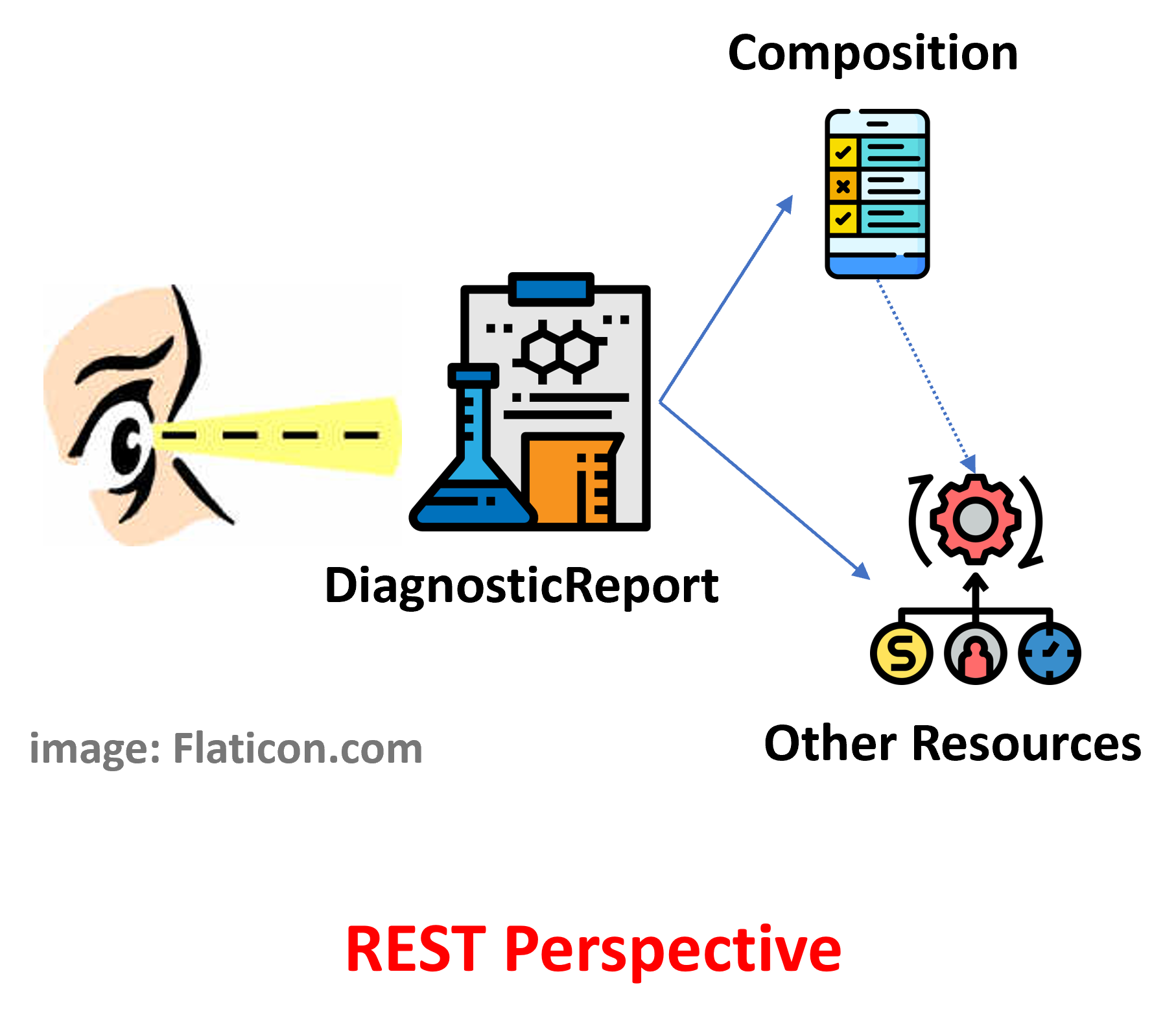
Figure 2 - Report as structured collection of results: DiagnosticReport with Composition
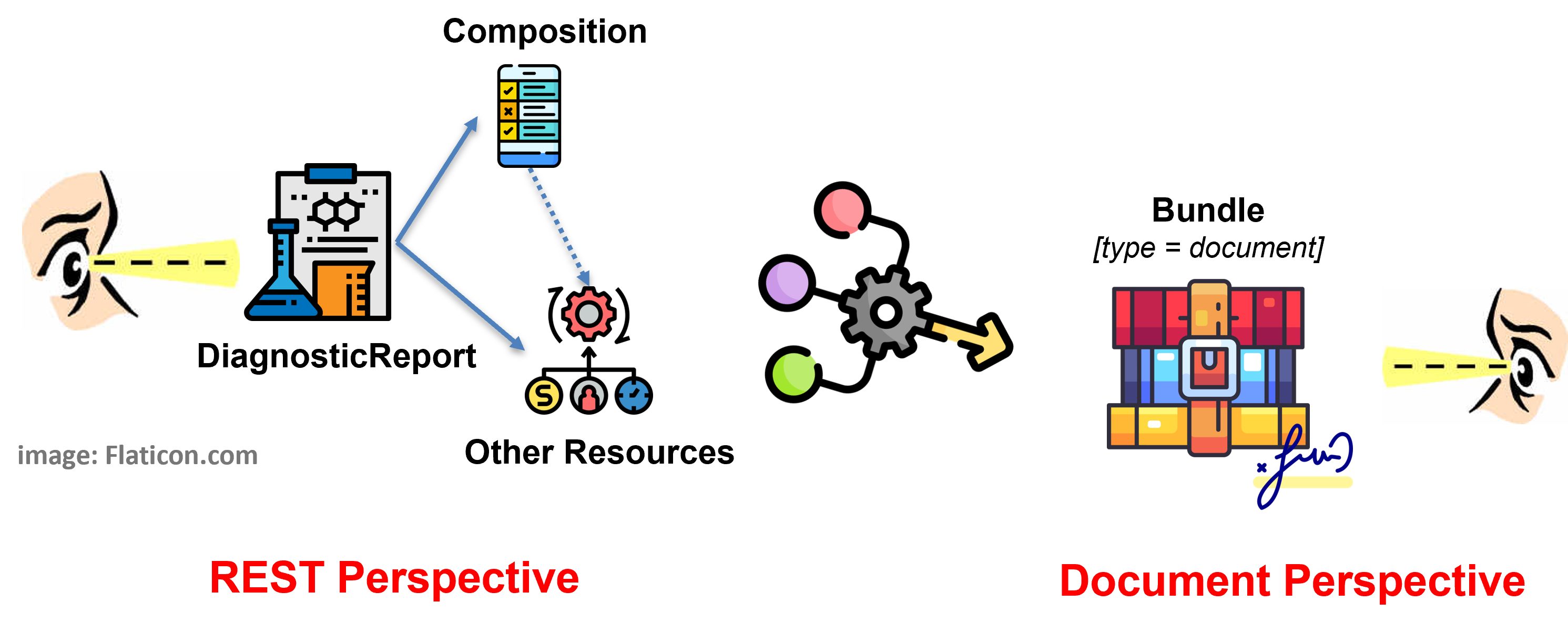
Figure 3 - Report as document: DiagnosticReport with Composition in a Document Bundle
The document based solution, adopted in the European realm, tries to balance the two expectations of having a HL7 FHIR document and searching reports via DiagnosticReport, limiting as much as possible the implementation options. Moreover it takes into account the R5 DiagnosticReport design pattern where the DiagnosticReport vs. Composition relationship is directed from the DiagnosticReport to the Composition resource.
The authors are aware of the fact that this choice requires additional work by the report creator, requesting to consistently record in both DiagnosticReport and Composition a set of information. However, they believe that it enables more options for the consumer:
To support the described documental approach, this guide allows for the pre-adoption of the R5 rules for the inclusion of the resources in a document Bundle, that is:
"The document bundle SHALL include only: <..> The supporting information: Any resources that are part of the graph of resources that reference or are referenced from the composition set, either directly or indirectly (e.g. recursively in a chain)"
in opposition to the R4 rules requiring that, with the exception of the Provenance resource and the Binary including the stylesheet, only resources directly or indirectly - referred from the composition can be included.
This choice is justified by the fact that:
However, this choice is not imposed, so that usage contexts wishing to keep a full consistency with R4 rules may use the DiagnosticReportReference extension to refer to the DiagnosticReport from the Composition. The adoption of this extension implies the presence of a circular reference of Composition to/from the DiagnosticReport.