This page is part of the Genetic Reporting Implementation Guide (v0.3.0: STU 1 Ballot 2) based on FHIR R4. The current version which supercedes this version is 2.0.0. For a full list of available versions, see the Directory of published versions 
This section provides guidance on reporting genetic tests that involve sequencing the DNA, RNA or amino acid chains of the specimen. This includes direct sequencing, shot-gun sequencing, array-based variant testing and other mechanisms.
There are three profiles provided for describing the results of genetic testing:
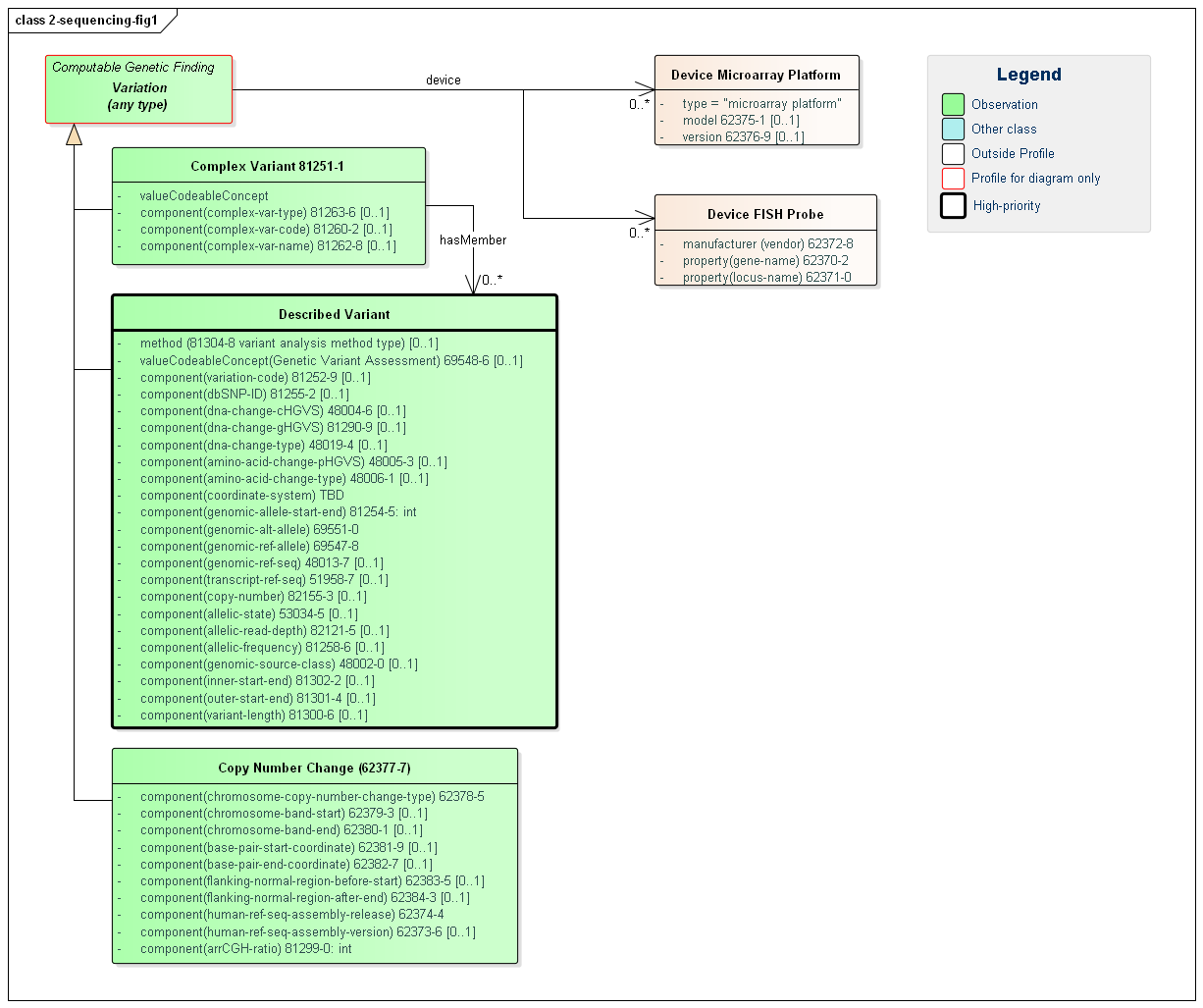
Figure 1: Sequencing Profiles
(Profile links: Complex Variant, Described Variant, Copy Number Change )
The core of the typical report is a list of identified variants – each with many possible attributes. Described Variant is a new term that HL7 has assigned to the notion of simple, discrete, or structural variant. We have done this because of the difficulty of drawing a clear line between these other labels for variants. The rules are often driven by the registries to which the variants are submitted and are not necessarily consistent. The difference may be driven by testing methodology rather than a difference in the underlying genetic characteristic.
This profile allows a full description of the variant found using properties from a variety of testing approaches and allowing for a variety of descriptive mechanisms. An individual variant may be defined and described by multiple attributes delivered as component values. Most of these components are optional, but labs are encouraged to populate what properties they know. Clinicians are accustomed to receiving descriptive information such as the HVGS genomic and amino acid expression, the type of variant (e.g. deletion) and the reference sequence. The identifiers to the online databases can be more easily consumed by CDS systems. In future versions of this implementation guide, HL7 may provide additional guidance on what properties should be sent in which situations and may subdivide Described Variant into multiple sub-profiles with more specific purpose.
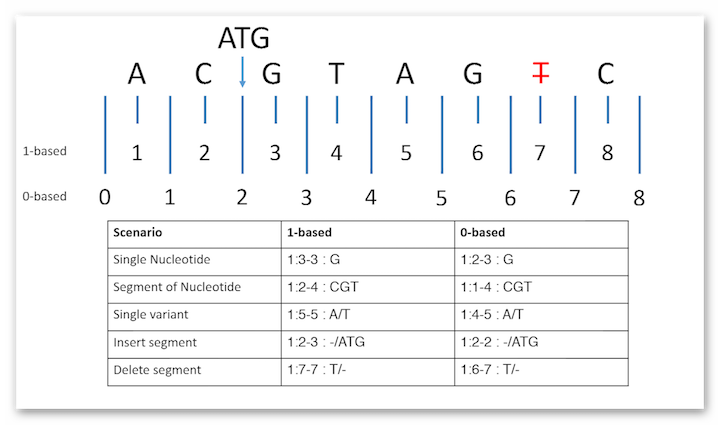
Note that there are two coordinate systems one can use when referencing positions within a molecular sequence. In particular, information from HGVS is 1-based, with special rules for consideration that can be less than straight forward when switching between insertions and deletions. For clarity and consistency, one must take care not to mix coordinate systems within the same resource instance. If HGVS is used within a resource instance, the rest of the instance should also be 1-based so as not to mix coordinate systems. Below is a picture that could explain the difference between the two systems:
The Complex Variant profile is used when there is a need to represent a named grouping of variants that often has a specified clinical effect or phenotype. An example would be a Compound heterozygote, but could be other types as found in the Complex Variant Type component that use this LOINC code. Many of the details of what was found are left to the member Described Variants the Complex Variant points to.
The Copy Number Change profile is used to document when a copy number variation has been detected. Using this profile, the type of copy number change can be represented, as well as additional information about the region impacted by the change.