This page is part of the Healthcare Associated Infection Reports (HAI) Long Term Care Facilities (LTCF) (v1.1.0: STU 1.1) based on FHIR R4. This is the current published version. For a full list of available versions, see the Directory of published versions 
| LinkId | Text | Definition | Answer |
|---|---|---|---|
 | QuestionnaireResponse | ||
 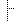 | Facility ID | urn:hl7ii:2.16.840.1.113883.3.117.1.1.5.1.1 | |
 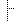 | First Day of Period Reported | 2023-01-01 | |
 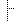 | Last Day of Period Reported | 2023-01-31 | |
 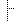 | Facility Location Code | NHSN Healthcare Facility Patient Care Location 1250-0: Facility-wide Inpatient (FacWIDEIn) | |
  | Summary Data Group | ||
   | Summary Data Group - Denominator | ||
    | Total Resident Days | 100 d | |
    | Urinary Catheter Days | 20 d | |
   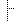 | New Antibiotic Starts for UTI Indication | 8 | |
   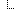 | Number of Urine Cultures Ordered | 2 | |
   | Summary Data Group - MDRO/CDI | ||
   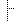 | Resident Days | 100 d | |
    | Resident Admissions | 20 | |
    | Number of admissions on C. difficile treatment | 8 | |
    | Number of C. difficile treatment starts | 2 | |
   | Summary Data Group - Prevention Process Measures | ||
    | Hand Hygiene Performed | 100 | |
    | Hand Hygiene Indicated | 20 | |
    | Gown and Gloves Used | 8 | |
    | Gown and Gloves Indicated | 2 | |
  | Report no events | ||
   | Report No Events: LabID Event (All specimens): VRE | true | |