This page is part of the FHIR Specification (v1.4.0: STU 3 Ballot 3). The current version which supercedes this version is 5.0.0. For a full list of available versions, see the Directory of published versions 
URL for this extension:
http://hl7.org/fhir/StructureDefinition/qicore-adverseevent-clinicalStudy
Status: draft. Extension maintained by: Health Level Seven International (Clinical Quality Information - QICore)
The NCT numbers of the clinical studies the patient participates in at the time of the adverse event (if available).
Context of Use: Use on element: Basic
usage info: insert a list of places where this extension is used
Summary
| Name | Flags | Card. | Type | Description & Constraints |
|---|---|---|---|---|
 | 0..* | string | URL = http://hl7.org/fhir/StructureDefinition/qicore-adverseevent-clinicalStudy Related clinical study: The NCT numbers of the clinical studies the patient participates in at the time of the adverse event (if available). Use on element: Basic | |
 Documentation for this format Documentation for this format | ||||
Fulle Structure
| Name | Flags | Card. | Type | Description & Constraints |
|---|---|---|---|---|
 | 0..* | Extension | URL = http://hl7.org/fhir/StructureDefinition/qicore-adverseevent-clinicalStudy Related clinical study: The NCT numbers of the clinical studies the patient participates in at the time of the adverse event (if available). Use on element: Basic | |
 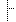 | 0..0 | |||
 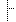 | 1..1 | uri | "http://hl7.org/fhir/StructureDefinition/qicore-adverseevent-clinicalStudy" | |
 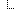 | 1..1 | string | Value of extension | |
 Documentation for this format Documentation for this format | ||||
XML Template
<!-- Related clinical study --><extension xmlns="http://hl7.org/fhir" url="http://hl7.org/fhir/StructureDefinition/qicore-adverseevent-clinicalStudy" > <!-- from Element: extension --> <valueString value="[string]"/><!-- 1..1 Value of extension --> </extension>
JSON Template
{ // Related clinical study
// from Element: extension
"url" : "http://hl7.org/fhir/StructureDefinition/qicore-adverseevent-clinicalStudy", // R!
"valueString" : "<string>" // R! Value of extension
}
Summary
| Name | Flags | Card. | Type | Description & Constraints |
|---|---|---|---|---|
 | 0..* | string | URL = http://hl7.org/fhir/StructureDefinition/qicore-adverseevent-clinicalStudy Related clinical study: The NCT numbers of the clinical studies the patient participates in at the time of the adverse event (if available). Use on element: Basic | |
 Documentation for this format Documentation for this format | ||||
Full Structure
| Name | Flags | Card. | Type | Description & Constraints |
|---|---|---|---|---|
 | 0..* | Extension | URL = http://hl7.org/fhir/StructureDefinition/qicore-adverseevent-clinicalStudy Related clinical study: The NCT numbers of the clinical studies the patient participates in at the time of the adverse event (if available). Use on element: Basic | |
 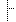 | 0..0 | |||
  | 1..1 | uri | "http://hl7.org/fhir/StructureDefinition/qicore-adverseevent-clinicalStudy" | |
  | 1..1 | string | Value of extension | |
 Documentation for this format Documentation for this format | ||||
XML Template
<!-- Related clinical study --><extension xmlns="http://hl7.org/fhir" url="http://hl7.org/fhir/StructureDefinition/qicore-adverseevent-clinicalStudy" > <!-- from Element: extension --> <valueString value="[string]"/><!-- 1..1 Value of extension --> </extension>
JSON Template
{ // Related clinical study
// from Element: extension
"url" : "http://hl7.org/fhir/StructureDefinition/qicore-adverseevent-clinicalStudy", // R!
"valueString" : "<string>" // R! Value of extension
}